Abstract
Introduction: The target activated partial thromboplastin time (aPTT) range of 1.5 to 2.5 times the control value or 45 to 75 seconds recommended by the ACC/AHA for patients receiving unfractionated heparin (UFH) for acute coronary syndromes (ACS) is vulnerable to variation in test reagents. Rather than use the standard target aPTT range, it has been recommended that each institution establish its own target aPTT range based upon anti-factor Xa heparin levels. As a quality assurance project, we evaluated our institution's therapeutic aPTT range by examining the correlation between aPTTs and anti-factor Xa heparin levels and established a new target aPTT range with a new thromboplastin reagent based upon the therapeutic anti-factor Xa heparin levels.
Methods: Sixty-two plasma samples from 26 consecutive patients receiving UFH for ACS were analyzed. Plasma aPTTs measured with a thromboplastin reagent and a new thromboplastin reagent and anti-factor Xa heparin levels were obtained on each plasma sample. Linear regression analysis was performed to establish a new target aPTT range from corresponding therapeutic anti-factor Xa heparin levels.
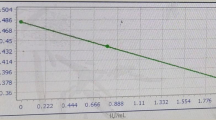
Results: Thirty-two percent of patients with our institution's target range aPTTs of 61 to 100 seconds had anti-factor Xa heparin levels below 0.35 to 0.7 U/mL while 68% of patients had therapeutic anti-factor Xa heparin levels (positive predictive value = 68%). When the same blood was tested with a new thromboplastin reagent lot, only 9% of patients with target range aPTTs had anti-factor Xa heparin levels below 0.35 U/mL while 91% of patients had therapeutic anti-factor Xa heparin levels (positive predictive value = 91%). The Pearson correlation coefficient (r) for the new thromboplastin reagent lot was 0.93. The target aPTT range established with the new thromboplastin reagent lot was 61 to 100 seconds.
Conclusion: Monitoring aPTTs without standardizing the thromboplastin reagent may not adequately reflect therapeutic heparin levels. Despite apparently target aPTTs, patients treated with UFH may be under-anticoagulated. Our new anti-Xa-adjusted target aPTT range shows an increase in the positive predictive value of aPTTs. Large-scale clinical studies are needed to determine the optimal anti-factor Xa range for ACS patients treated with UFH, with further refinements if glycoprotein IIb/IIIa inhibitors are concomitantly used and to show a benefit in clinical outcomes for monitoring plasma heparin levels with anti-factor Xa heparin levels. Institutional standardization of the aPTT is necessary to ensure optimal patient care when changing thromboplastin reagents.
Similar content being viewed by others
References
Ambrose JA, Winters SL, Arora RR, et al. Angiographic evaluation of coronary artery morphology in unstable angina. J AmColl Cardiol 1986;7:474–478.
Williams AE, Freeman MR, Chisolm RJ, Patt NL, Armstrong PW. Angiographic morphology in unstable angina pectoris. Am J Cardiol 1988;62:1024–1027.
Falk E, Shah PK, Fuster V. Coronary plaque disruption. Circulation 1995;92:657–671.
Fuster V, Badimon L, Badimon JJ, Cheseboro JH. The pathogenesis of coronary artery disease and the acute coronary syndromes (2). N Eng J Med 1992;36:310–318.
Vetrovec G, Leinback R, Gold HK, Cowley MJ. Intracoronary thrombolysis in syndromes of unstable angina: Angiographic and clinical results. Am Heart J 1982;104:946–952.
Mandelkorn J, Wolf N, Singh S, et al. Intracoronary thrombus in nontransmural myocardial infarction and in unstable angina pectoris. Am J Cardiol 1983;52:1–6.
Sherman CT, Litvack F, Gundfest T, et al. Coronary angioscopy in patients with unstable angina pectoris. N Eng J Med 1986;315:913–919.
Mizuno K, Satomora K, Miyamoto A, et al. Coronary angioscopy in patients with unstable angina pectoris. N Engl J Med 1992;326:287–291.
Theroux P, Ouimet H, McCans J, et al. Aspirin, heparin, or both o treat acute unstable angina. N Engl J Med 1988;319:1105–1111.
The PURSUIT Trial Investigators. Inhibition of platelet glycoprotein IIb/IIIa with eptifibatide in patients with acute coronary syndromes. N Engl J Med 1998;339:436–443.
The PRISM Study Investigators. A comparison of aspirin plus tirofiban with aspirin plus heparin for unstable angina. N Engl J Med 1998;338:1498–1505.
The PRISM-PLUS Study Investigators. Inhibition of the platelet glycoprotein IIb/IIIa receptor with tirofiban in unstable angina and non-Q-wave myocardial infarction}. N Engl J Med 1998;338:1488–1497.
Gawoski J, Arkin CF, Bovill T, Brandt JT, Rock WA Jr, Triplett DA. The effects of heparin on the activated partial thromboplastin time of the College of American Pathologists survey specimens: Responsiveness, precision, and sample effects. Arch Pathol Lab Med 1987;111:785–790.
Braunwald E, Antman EM, Beasley JW, et al. ACC/AHA guidelines for the management of patients with unstable angina, non-ST-segment elevation myocardial infarction. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines 126 Lee et al. (Committee of the Management of Patients with Unstable Angina). J AmColl Cardiol 2000;36:970–1062.
Gawoski J, Arkin CF, Bovill T, et al. The effects of heparin on the activated partial thromboplastin time of the College of American Pathologists survey specimens: Responsiveness, precision, and sample effects. Arch Pathol Lab Med 1987;111:785–790.
Bain B, Forster T, Sleigh B. Heparin and the activated partial thromboplastin time-a difference between the in-vitro and in-vivo effects and implications for the therapeutic range. Am J Clin Pathol 1980;74:668–673.
Zanke B, Shojania AM. Comparison of two APTT methods of monitoring heparin therapy. APTT ratio and heparin response of pooled normal plasma. Am J Clin Pathol 1990;93:684–689.
Scalla SJ. Heparin monitoring by activated partial thromboplastin time. Comparison of ex vivo measurement and in vitro standardization. Am J Clin Pathol 1985;84:351–354.
van den Besselaar AM, Meeuwisse-Braun J, Bertina RM. Monitoring heparin therapy: Relationships between the activated partial thromboplastin time and heparin assays based on ex-vivo heparin samples. Thromb Haemost 1990;63:16–23.
Shojania AM, Tetreault J, Turnbull G. The variations between heparin sensitivity of different lots of activated partial thromboplastin time reagent produced by the same manufacturer. Am J Clin Pathol 1988;89:19–23.
Poller L, Hirsh J. Special report: A simple system for the derivation of international normalized ratios for the reporting of prothrombin time results with North American thromboplastin reagents. Am J Clin Pathol 1989;92:124–1266.
Braunwald E. Mark DB, Jones RH, et al. Unstable angina: Diagnosis and management. Clinical Practice Guideline No. 10. Rockville, MD: Agency for Health Care Policy and Research, 1994. (AHCPR Publication No. 94-0602).
Theroux P, Ouimet H, McCans J, et al. Aspirin, heparin or both to treat unstable angina. N Eng J Med 1988;319:1105–1111.
Theroux P, Waters D, Qui S, McCans J, deGuise P, Juneau M. Aspirin versus heparin to prevent myocardial infarction during the acute phase of unstable angina. Circulation 1993;88:2045–2048.
The RISC Group. Risk of myocardial infarction and death during treatment with low dose aspirin and intravenous heparin in men with unstable coronary artery disease. Lancet 1990;336:827–830.
Cohen M, Adams PC, Parry G, et al. Combination antithrombotic therapy in unstable rest angina and non-Q-wave infarction in non-prior aspirin users: Primary end points analysis from the ATACS trial. Circulation 1994;89:81–88.
Fragmin during Instability in Coronary Artery Disease (FRISC) Study Group. Low-molecular-weight heparin during instability in coronary artery disease. Lancet 1996;347:561–568.
Oler A, Whooley MA, Oler J, Grady D. Adding heparin to aspirin reduces the incidence of myocardial infarction and death in patients with unstable angina: A meta-analysis. JAMA 1996;276:811–815.
Brill-Edwards P, Ginsberg JS, Johnston M, Hirsh J. Establishing a therapeutic range for heparin therapy. Ann Intern Med 1993;119:104–109.
Hirsch J, Warkentin TE, Shaughnessy SG, et al. Heparin and low-molecular-weight heparin: Mechanisms of action, pharmacokinetics, dosing, monitoring, efficacy, and safety. Sixth ACCP Consensus Conference on Antithrombotic Therapy. Chest 2001; 119:64S–93S.
Menon V, Berkowitz SD, Antman EM, Fuchs RM, Hochman JS. New heparin dosing recommendations for patients with acute coronary syndromes. Am J Med 2001;110:641–650.
Bates SM, Weitz JI, Johnston M, Hirsch J, Ginsberg JS. Use of a fixed activated partial thromboplastin time ratio to establish a therapeutic range of unfractionated heparin. Arch Intern Med 2001;161:385–391.
Mesters RM, Mikoteit T, Schiller M, Krings W, Ostermann H, Kienast J. Markers of coagulation activation for evaluation of the antithrombotic efficacy of heparin: A prospective study in acute deep venous thrombosis. Blood Coagul Fibrinolysis 1995;6:665–671.
Young E, Pruis M, Levine MN, et al. Heparin building to plasma proteins: An important mechanism for heparin resistance. Thromb Haemost 1992;67:639–643.
Hirsh J, Van Aken WG, Gallus AS, Dollery CT, Cade JF, Yung WL. Heparin kinetics in venous thrombosis and pulmonary embolism. Circulation 1976;53:691–695.
Levine MN, Hirsh J. Gent M, et al. A randomized trial comparing activated thromboplastin time with heparin assay in patients with acute venous thromboembolism requiring large daily doses of heparin. Arch Intern Med 1994;154:49–56.
Aiach M, Michaud A, Balian JL, et al. A new low molecular weight heparin derivative, in vitro and in vivo studies. Thromb Res 1983;31:611–621.
Bara L, Billand E, Gramond G, et al. Comparative pharmacokinetics of a low molecular weight heparin (PK 10 169) and unfractionated heparin after intravenous and subcutaneous administration. Thromb Res 1985;39:631–636.
Turpie AGG. Can we differentiate the low-molecular weight heparins? Clin Cardiol 2000;23(Suppl I):14–17.
Cohen M, Demers C, Gurfinkel EP, et al. A comparison of low-molecular-weight heparin with unfractionated heparin for unstable coronary artery disease: Efficacy and Safety of Subcutaneous Enoxaparin in Non-Q-Wave Coronary Events Study Group. N Engl J Med 1997;337:447–452.
Antman EM, McCabe CH, Gurfinkel EP, et al. Enoxaparin prevents death and cardiac ischemic events in unstable angina/non-Q-wave myocardial infarction: Results of the Thrombolysis in Myocardial Infarction (TIMI) 11B. Circulation 1999;100:1593–1601.
Basu D, Gallus A, Hirsh J, Cade J. A prospective study of the value of monitoring heparin treatment with the activated partial thromboplastin time. N Engl J Med 1972;287:324–327.
Chiu HM, Hirsh J, Yung WL, Regoeczi E, Gent M. Relationship between the anticoagulant and antithrombotic effects of heparin in experimental venous thrombosis. Blood 1977;49:171–184.
Levine MN, Hirsh J, Gent, et al. A randomized trial comparing activated thromboplastin time with heparin assay in patients with acute venous thromboembolism requiring large daily doses of heparin. Arch Intern Med 1994;154:49–56.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lee, M.S., Menon, V., Schappert, J. et al. Establishing a New Target Range for Unfractionated Heparin for Acute Coronary Syndromes. J Thromb Thrombolysis 17, 121–126 (2004). https://doi.org/10.1023/B:THRO.0000037667.52940.64
Issue Date:
DOI: https://doi.org/10.1023/B:THRO.0000037667.52940.64