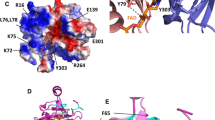
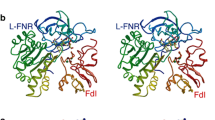
Abstract
Electron transfer (ET) reactions in systems involving proteins require an oriented interaction between electron donor and acceptor in order to accommodate their respective redox centres in optimal orientation for efficient ET. Such type of reactions are critical for the maintenance of the physiological functions of living organisms, since they are implicated in vital actions, as is, for example, in the photosynthetic ET chain that leads to NADPH reduction. In this particular case, a small redox protein ET chain is responsible for ET from Photosystem I (PS I) to NADP+. In this system the enzyme responsible for NADP+ reduction is ferredoxin–NADP+ reductase (FNR), a FAD-containing NADP+ dependent reductase. In order to produce such reduction, this enzyme receives electrons from a [2Fe–2S] plant-type ferredoxin (Fd), which is previously reduced by PS I. Moreover, in the case of some algae and cyanobacteria, an FMN-dependent protein, flavodoxin (Fld), has been shown to replace Fd in this function. The processes of interaction and ET between FNR and all of its substrates involved in the photosynthetic ET chain, namely Fd, Fld and NADP+/H have been extensively investigated in recent years using a large number of techniques, including the introduction of site-specific mutations in combination with kinetic and structural studies of the produced mutants. The present manuscript summarises the information so far reported for an efficient interaction between FNR and its substrates, compares such information with that revealed by other systems for which the FNR structure is a prototype and, finally, discusses the implications of the processes of association in ET between FNR and its substrates.
Similar content being viewed by others
References
Aliverti A, Gadda G, Ronchi S and Zanetti G (1991a) Identification of Lys116 as the target of N-ethylmaleimide inactivation of ferredoxin:NADP+ oxidoreductase. Eur J Biochem 198: 21–24
Aliverti A, Lübberstedt T, Zanetti G, Herrmann TG and Curti B (1991b) Probing the role of lysine 116 and lysine 224 in the spinach ferredoxin–NADP+ reductase by site-directed mutagenesis.J Biol Chem 266: 17760–17763
Aliverti A, Corrado ME and Zanetti G (1994) Involvement of lysine-88 of spinach ferredoxin–NADP+ reductase in the interaction with ferredoxin. FEBS Lett 343: 247–250
Aliverti A, Bruns CM, Pandini VE, Karplus PA, Vanoni MA, Curti B and Zanetti G (1995) Involvement of serine 96 in the catalytic mechanism of spinach ferredoxin–NADP+ reductase: structure– function relationship as studied by site-directed mutagenesis and X-ray crystallography. Biochemistry 34: 12842–12848
Aliverti A, Deng Z, Ravasi D, Piubelli L, Karplus PA and Zanetti G (1998) Probing the function of the invariant glutamyl residue 312 in spinach ferredoxin–NADP+ reductase. J Biol Chem 273: 34008–34015
Avron M and Jagendorf AT (1956) A TPNH diaphorase from chloroplasts. Arch Biochem Biophys 65: 475–490
Avron M and Jagendorf AT (1957) Some further investigations on chloroplasts TPNH diaphorase. Arch Biochem Biophys 72: 17–24
Batie CJ and Kamin H (1984a) Ferredoxin NADP+ oxidoreductase.Equilibria in binary and ternary complexes with NADP+ and ferredoxin. J Biol Chem 259: 8832–8839
Batie CJ and Kamin H (1984b) Electron transfer by ferredoxin NADP+ oxidoreductase. Rapid-reaction evidence for participation of a ternary complex. J Biol Chem 259: 11976–11985
Batie CJ and Kamin H (1986) Association of ferredoxin NADP+ reductase with NADP+(H). Specificity and oxidation–reduction properties. J Biol Chem 261: 11214–11223
Bernhardt R (2000) The role of adrenodoxin in adrenal steroidogenesis.Curr Opin End Diab 7: 109–115
Bhattacharyya AK, Meyer TE and Tollin G (1986) Reduction kinetics of the ferredoxin–ferredoxin–NADP+ reductase complex: a laser flash photolysis study. Biochemistry 25: 4655–4661
Bruns CM and Karplus PA (1995) Refined crystal structure of spin-achferredoxin reductase at 1.7 Åresolution: oxidized, reducedand 2′-phospho-5′-AMP bound states. J Mol Biol 247: 125–145
Campbell WH (1999) Nitrate reductase structure, function and regulation: bridging the gap between biochemistry and physiology.Annu Rev Plant Physiol Plant Mol Biol 50: 277–303
Carrillo N and Ceccarelli EA (2003) Open questions in ferredoxin– NADP+ reductase catalytic mechanism. Eur J Biochem 270: 1900–1915
Casaus JL, Navarro JA, Hervás M, Lostao A, De la Rosa MA, Gómez-Moreno C, Sancho J and Medina M (2002) Anabaena sp. PCC 7119 flavodoxin as electron carrier from Photosystem I to ferredoxin–NADP+ reductase. Role of Trp57 and Tyr94. J Biol Chem 277: 22338–22344
Chan RL, Carrillo N and Vallejos RH (1985) Isolation and sequencing of an active-site peptide from spinach ferredoxin–NADP+ oxidoreductase after affinity labeling with periodate-oxidized NADP+. Arch Biochem Biophys 240: 172–177
Cidaria D, Biondi PA, Zanetti G and Ronchi S (1985) The NADP+-binding site of ferredoxin–NADP+ reductase. Sequence of the peptide containing the essential lysine residue. Eur J Biochem 146: 295–299
Correll CC, Batie CJ, Ballou DP and Ludwig ML (1992) Phtalate dioxygenase reductase: a modular structure for electron transfer from pyridine nucleotides to [2Fe–2S]. Science 258: 1604–1610
Crowley PB and Ubbink M (2003) Close encounters of the transient kind: protein interactions in the photosynthetic redox chain investigated by NMR spectroscopy. Acc Chem Res 36: 723–730
DeLano WL, Ultsch MH, de Vos AM and Wells JA (2000) Convergent solutions to binding at a protein–protein interface. Science 287: 1279–1283
Deng Z, Aliverti A, Zanetti G, Arakaki AK, Ottado J, Orellano EG, Calcaterra NB, Ceccarelli EA, Carrillo N and Karplus PA (1999) A productive NADP+ binding mode of ferredoxin–NADP+ reductase revealed by protein engineering and crystallographic studies. Nat Struct Biol 6: 847–853
Ejdebäck M, Bergkvist A, Karlsson BG and Ubbink M (2000) Sidechain interactions in the plastocyanin–cytochrome f complex.Biochemistry 39: 5022–5027
Faro M, Gómez-Moreno C, Stankovich M and Medina M (2002a) Role of critical charged residues in reduction potential modulation of ferredoxin–NADP+ reductase. Differential stabilization of FAD redox forms. Eur J Biochem 269: 2656–2661
Faro M, Frago S, Mayoral T, Hermoso JA, Sanz-Aparicio J, Gómez-Moreno C and Medina M (2002b) Probing the role of the glutamic 139 residue in Anabaena ferredoxin–NADP+ reductase in its interaction with substrates. Eur J Biochem 269: 4938–4947
Foust GP, Mayhew SG and Massey V (1969) Complex formation between ferredoxin triphosphopyridine nucleotide reductase and electron transfer proteins. J Biol Chem 244: 964–970
Gómez-Moreno C, Martínez-JÚlvez M, Medina M, Hurley JK and Tollin G (1998) Protein–protein interaction in electron transfer reactions: the ferredoxin/flavodoxin/ferredoxin–NADP+ reductase system from Anabaena. Biochimie 80: 837–846
Gruez A, Pignol D, Zeghouf M, Covès J, Fontecave M, Ferrer J-L and Fontecilla-Camps JC (2000) Four crystal structures of the 60 kDa flavoprotein monomer of the sulfite reductase indicate a disordered flavodoxin-like module. J Mol Biol 299: 199–212
Hermoso JA, Mayoral T, Faro M, Gómez-Moreno C, Sanz-Aparicio J and Medina M (2002) Mechanism of coenzyme recognition and binding revealed by crystal structure analysis of ferredoxin– NADP+ reductase complexed with NADP+. J Mol Biol 319: 1133–1142
Hunter CA and Sanders JKM(1990) The nature of π–π interactions.J Am Chem Soc 112: 5525–5534
Hurley JK, Salamon Z, Meyer TE, Fitch JC, Cusanovich MA, Markley JL, Cheng H, Xia B, Chae YK, Medina M, Gómez-Moreno C and Tollin G (1993a) Amino acid residues in Anabaena ferredoxin crucial to interaction with ferredoxin–NADP+ reductase: site-directed mutagenesis and laser flash photolysis.Biochemistry 32: 9346–9354
Hurley JK, Cheng H, Xia B, Markley JL, Medina M, Gómez-Moreno C and Tollin G (1993b) An aromatic amino acid is required at position 65 in Anabaena ferredoxin for rapid electron transfer to ferredoxin–NADP+ reductase. J Am Chem Soc 115: 11698–11701
Hurley JK, Medina M, Gómez-Moreno C and Tollin G (1994) Further characterization by site-directed mutagenesis of the protein– protein interface in the ferredoxin/ferredoxin:NADP+ reductase system from Anabaena: requirement of a negative charge at position 94 in ferredoxin for rapid electron transfer. Arch Biochem Biophys 312: 480–486
Hurley JK, Weber-Main AM, Stankovich MT, Benning MM, Thoden JB, Vanhooke JL, Holden HM, Chae YK, Xia B, Cheng H, Markley JL, Martínez-JÚlvez M, Gómez-Moreno C, Schmeits JL and Tollin G (1997) Structure–function relationships in Anabaena ferredoxin: correlations between X-ray crystal structures, reduction potentials, and rate constants of electron transfer to ferredoxin–NADP+ reductase for site-specific ferredoxin mutants. Biochemistry 36: 11100–11117
Hurley JK, Faro M, Brodie TB, Hazzard JT, Medina M, Gómez-Moreno C and Tollin G (2000) Highly non-productive complexes with Anabaena ferredoxin at low ionic strength are induced by non-conservative amino acid substitution at Glu139in Anabaena ferredoxin–NADP+ reductase. Biochemistry 39: 13695–13702
Ingelman M, Bianchi V and Eklund H (1997) The three-dimensional structure of flavodoxin reductase from Escherichia coli at 1.7Å resolution. J Mol Biol 268: 147–157
Ingelman M, Ramaswamy S, Nivière V, Fontecave M and Eklund H (1999) Crystal structure of NAD(P)H: flavin oxidoreductase from Escherichia coli. Biochemistry 38: 7040–7049
Jelesarov I and Bosshard HR (1994) Thermodynamics of ferredoxin binding to ferredoxin:NADP+ reductase and the role of water at the complex interface. Biochemistry 33: 13321–13328
Jelesarov I, De Pascalis AR, Koppenol WH, Hirasawa M, Knaff DB and Bosshard R (1993) Ferredoxin binding site on ferredoxin– NADP+ reductase. Differential chemical modification of free and ferredoxin bound enzyme. Eur J Biochem 216: 57–66
Jenkins CM, Genzor CG, Fillat MF, Waterman MR and Gómez-Moreno C (1997) Negatively charged Anabaena flavodoxin residues (Asp144 and Glu145) are important for reconstitution of cytochrome P450 17-alpha-hydroxylase activity. J Biol Chem 36: 22509–22513
Jung Y-S, Roberts VA, Stout CD and Burgess BK (1999) Complex formation between Azotobacter vinelandii ferredoxin and its physiological electron donor NADPH–ferredoxin reductase. J Biol Chem 274: 2978–2987
Karplus PA and Bruns M (1994) Structure–function relations for ferredoxin reductase. J Bioenerg Biobr 26: 89–99
Karplus PA, Daniels MJ and Herriot JR (1991) Atomic structure of ferredoxin–NADP+ reductase: prototype for a structurally novel flavoenzyme family. Science 251: 60–66
Kurisu G, Kusunoki M, Katoh E, Yamazaki T, Teshima K, Onda Y, Kimata-Ariga Y and Hase T (2001) Structure of the electron transfer complex between ferredoxin and ferredoxin–NADP+ reductase. Nat Struct Biol 8: 117–121
Liocher SI, Hausladen A, Beyer WF and Fridovich I (1994) NADPH:ferredoxin oxidoreductase acts as a paraquat diaphorase and is a member of the soxRS regulon. Proc Natl Acad Sci USA 91: 1328–1331
Lostao A, Gómez-Moreno C, Mayhew SG and Sancho J (1997) Differential stabilization of the three FMN redox forms by tyrosine 94 and tryptophan 57 in flavodoxin from Anabaena and its influence on the redox potentials. Biochemistry 36: 14334–14344
Lu G, Campbell WH, Schneider G and Lindqvist Y (1994) Crystal structure of the FAD-containing fragment of maize nitrate reductase at 2.5Å resolution: relationship to other flavoprotein reductases. Structure 2: 809–821
Martínez-JÚlvez M, Medina M, Hurley JK, Hafezi R, Brodie T, Tollin G and Gómez-Moreno C (1998a) Lys75 of Anabaena ferredoxin–NADP+ reductase is a critical residue for binding ferredoxin and flavodoxin during electron transfer. Biochemistry 37: 13604–13613
Martínez-JÚlvez M, Hermoso JA, Hurley JK, Mayoral T, Sanz-Aparicio J, Tollin G, Gómez-Moreno C and Medina M (1998b) Role of Arg100 and Arg264 from Anabaena PCC7119ferredoxin–NADP+ reductase for optimal binding and electron transfer. Biochemistry 37: 17680–17691
Martínez-JÚlvez M, Medina M and Gómez-Moreno C (1999) Ferredoxin–NADP+ reductase uses the same site for the interaction with ferredoxin and flavodoxin. J Biol Inorg Chem 4: 568–578
Martínez-JÚlvez M, Nogués I, Faro M, Hurley JK, Brodie TB, Mayoral T, Sanz-Aparicio J, Hermoso JA, Stankovich MT, Medina M, Tollin G and Gómez-Moreno C (2001) Role of a cluster of hydrophobic residues near the FAD cofactor in Anabaena PCC7119 ferredoxin–NADP+ reductase for optimal complex formation and electron transfer to ferredoxin. J Biol Chem 276: 27498–27510
Maté MJ, Ortiz-Lombardin M, Boitel B, Haouz A, Tello D, Susin SA, Penninger J, K roemer G and Alzari P (2002) The crystal structure of mouse apoptosis-inducing factor AIF. Nat Struct Biol 9: 442–446
Mayoral T, Medina M, Sanz-Aparicio J, Gómez-Moreno C and Hermoso JA (2000) Structural basis of the catalytic role of Glu301 in Anabaena PCC 7119 ferredoxin–NADP+ reductase revealed by X-ray crystallography. Proteins 38: 60–69
Medina M, Mendez E and Gómez-Moreno C (1992a) Identification of arginyl residues involved in the binding of ferredoxin– NADP+ reductase from Anabaena sp. PCC 7119 to its substrates. Arch Biochem Biophys 299: 281–286
Medina M, Mendez E and Gómez-Moreno C (1992b) Lysine residues on ferredoxin–NADP+ reductase from Anabaena sp.PCC 7119 involved in substrate binding. FEBS Lett 298: 25–28
Medina M, Peleato ML, Mendez E and Gómez-Moreno C (1992c) Identification of specific carboxyl groups on Anabaena PCC 7119 flavodoxin which are involved in the interaction with ferredoxin–NADP+ reductase. Eur J Biochem 203: 373–379
Medina M, Martínez-JÚlvez M, Hurley JK, Tollin G and Gómez-Moreno C (1998) Involvement of glutamic acid 301 in the catalytic mechanism of ferredoxin–NADP+ reductase from Anabaena PCC7119. Biochemistry 37: 2715–2728
Medina M, Luquita A, Tejero J, Hermoso J, Mayoral M, Sanz-Aparicio J, Grever K and Gómez-Moreno C (2001) Probing the determinants of coenzyme specificity in ferredoxin–NADP+ reductase by site-directed mutagenesis. J Biol Chem 276: 11902–11912
Morales R, Charon MH, Hudry-Clergeon G, Petillot Y, Norager S, Medina M and Frey M (1999) Refined X-ray structures of the oxidized, at 1.3 Å, and reduced, at 1.17Å, [2Fe–2S] ferredoxin from the cyanobacterium Anabaena PCC7119 show redox-linked conformational changes. Biochemistry 38: 15764– 15773
Morales R, C haron M-H, Kachalova G, Serre L, Medina M, Gómez-Moreno C and Frey M (2000) A redox-dependent interaction between two electron-transfer partners involved in photosynthesis.EMBO Rep 1: 271–276
Navarro JA, Hervás M, G enzor CG, Cheddar G, Fillat MF, de la Rosa MA, Gómez-Moreno C, Cheng H, Xia B, Chae YK, Yan H, Wong B, Straus A, Markley JL, Hurley JK and Tollin G (1995) Site-specific mutagenesis demonstrates that the structural requirements for efficient electron transfer in Anabaena ferredoxin and flavodoxin are highly dependent on the reaction partner: kinetic studies with Photosystem I, ferredoxin:NADP+ reductase, and cytochrome c. Arch Biochem Biophys 321: 229–238
Nishida H, Inaka K, Yamanaka M, Kaida S, Kobayashi K and Miki K (1995) Crystal structure of NADH–cytochrome b5 reductase from pig liver at 2.4Å resolution. Biochemistry 34: 273–2767
Nogués I, Martínez-JÚlvez M, Navarro JA, Hervás M, Armenteros L, de la Rosa MA, Brodie TB, Hurley JK, Tollin G, Gómez-Moreno C and Medina M (2003) Role of hydrophobic interactions in the flavodoxin mediated electron transfer from Photosystem I to ferredoxin–NADP+ reductase in Anabaena PCC 7119. Biochemistry 42: 2036–2045
Piubelli L, Aliverti A, Arakaki AK, Carrillo N, Ceccarelli EA, Karplus PA and Zanetti G (2000) Competition between Cterminal tyrosine and nicotinamide modulates pyridine nucleotide affinity and specificity in plant ferredoxin–NADP+ reductase. J Biol Chem 275: 10472–10476
Poulos TL, Finzel BC and Howard AJ (1986) Crystal structure of substrate-free Pseudomonas putida cytochrome P-450. Biochemistry 25: 5314–5322
Prasad GS, Kresge N, Muhlberg AB, Shaw A, Jung YS, Burgess BK and Stout CD (1998) The crystal structure of NADPH:ferredoxin reductase from Azotobacter vinelandii. Protein Sci 7: 2541–2549
Pueyo JJ and Gómez-Moreno C (1991) Purification of ferredoxin NADP+ reductase from Anabaena PCC7119. Prep Biochem 21: 191–204
Pueyo JJ, Gómez-Moreno C and Mayhew SG (1991) Oxidation– reduction potentials of ferredoxin NADP+ reductase from Anabaena PCC7119 and of their electrostatic and covalent complexes. Eur J Biochem 202: 1065–1071
Rao ST, Shaffie F, Yu C, Satyshur KA, Stockman BJ, Markley JL and Sundaralingam M (1992) Structure of the oxidized longchain flavodoxin from Anabaena 7120 at 2Å resolution. Protein Sci 1: 1413–1427
Rypniewski WR, Breiter DR, Benning MM, Wasenberg G, Oh B-H, Markley JL, Rayment I and Holden HM (1991) Crystallisation and structure determination to 2.5Å resolution of the oxidised (2Fe–2S) ferredoxin isolated from Anabaena 7120.Biochemistry 30: 4126–4131
Senda T, Yamada T, Sakurai N, Kubota M, Nishizaki T, Masai E, Fukuda M and Mitsuidagger Y (2000) Crystal structure of NADH-dependent ferredoxin reductase component in biphenyl dioxygenase. J Mol Biol 304: 397–410
Serre L, Vellieux FMD, Medina M, Gómez-Moreno C, Fontecilla-Camps JC and Frey M (1996) X-ray structure of the ferredoxin:NADP+ reductase from the cyanobacterium Anabaena PCC 7119 at 1.8Å resolution, and crystallographic studies of NADP+ binding at 2.25Å resolution. J Mol Biol 263: 20–39
Shin M, T agawa K and Arnon DI (1963) Crystallization of ferredoxin–TPN reductase and its role in the photosynthetic apparatus of chloroplasts. Biochem Z 238: 84–86
Sridhar PG, Kresge N, Muhlberg AB, Shaw A, Jung YS, Burgress BK and Stout CD (1998) The crystal structure of NADPH:ferredoxin reductase from Azotobacter vinelandii. Protein Sci 7: 2541–2549
Susor WA and Krogmann DW (1966) Triphosphopyridine nucleotide photoreduction with cell-free preparations of Anabaena variabilis. Biochim Biophys Acta 120: 65–72
Ullmann GM, Hauswald M, Jensen A and Knapp EW (2000) Structural alignment of ferredoxin and flavodoxin based on electrostatic potentials: implications for their interactions with Photosystem I and ferredoxin–NADP+ reductase. Proteins 38: 301–309
Walker MC, Pueyo JJ, Gómez-Moreno C and Tollin G (1991) Comparison of the kinetics of reduction and intramolecular electron transfer in electrostatic and covalent complexes of ferredoxin– NADP+ reductase and flavodoxin from Anabaena PCC7119. Arch Biochim Biophys 281: 76–83
Wan JT and Jarrett JT (2002) Electron acceptor specificity of ferredoxin (flavodoxin): NADP+ oxidoreductase from Escherichia coli. Arch Biochem Biophys 406: 116–126
Wang M, Roberts DL, Paschke R, Shea TM, Masters BS and Kim JJ (1997) Three-dimensional structure of NADPH– cytochrome P450 reductase: prototype for FMN-and FAD-containing enzymes. Proc Natl Acad Sci USA 94: 8411–8416
Zanetti G, Gozzer C, Sacchi G and Curti B (1979) Modification of arginyl residues in ferredoxin–NADP+ reductase from spinach leaves. Biochim Biophys Acta 568: 127–134
Zanetti G, Aliverti A and Curti B (1984) A cross-linked complex between ferredoxin and ferredoxin–NADP+ reductase. J Biol Chem 259: 6153–6157
Ziegler GA and Schulz G (2000) Crystal structures of adrenodoxin reductase in complex with NADP+ and NADPH suggesting a mechanism for the electron transfer of an enzyme family.Biochemistry 39: 10986–10995
Ziegler GA, Vonrhein C, Hanukoglu I and Schulz G (1999) The structure of adrenodoxin reductase of mitochondrial P450 systems: electron transfer for steroid biosynthesis. J Mol Biol 289: 981–990
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Medina, M., Gómez-Moreno, C. Interaction of Ferredoxin–NADP+ Reductase with its Substrates: Optimal Interaction for Efficient Electron Transfer. Photosynthesis Research 79, 113–131 (2004). https://doi.org/10.1023/B:PRES.0000015386.67746.2c
Issue Date:
DOI: https://doi.org/10.1023/B:PRES.0000015386.67746.2c