Abstract
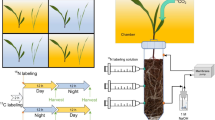
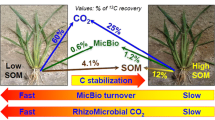
The effects of different shading periods of maize plants on rhizosphere respiration and soil organic matter decomposition were investigated by using a 13C natural abundance and 14C pulse labeling simultaneously. 13C was a tracer for total C assimilated by maize during the whole growth period, and 14C was a tracer for recently assimilated C. CO2 efflux from bare soil was 4 times less than the total CO2 efflux from planted soil under normal lighting. Comparing to the normal lighting control (12/12 h day/night), eight days with reduced photosynthesis (12/36 h day/night period) and strongly reduced photosynthesis (12/84 h day/night period) resulted in 39% and 68% decrease of the total CO2 efflux from soil, respectively. The analysis of 13C natural abundance showed that root-derived CO2 efflux accounted for 82%, 68% and 56% of total CO2 efflux from the planted soil with normal, prolonged and strongly prolonged night periods, respectively. Clear diurnal dynamics of the total CO2 efflux from soil with normal day-night period as well as its strong reduction by prolonged night period indicated tight coupling with plant photosynthetic activity. The light-on events after prolonged dark periods led to increases of root-derived and therefore of total CO2 efflux from soil. Any factor affecting photosynthesis, or substrate supply to roots and rhizosphere microorganisms, is an important determinant of root-derived CO2 efflux, and thereby, total CO2 efflux from soils. 14C labeling of plants before the first light treatment did not show any significant differences in the 14CO2 respired in the rhizosphere between different dark periods because the assimilate level in the plants was high. Second labeling, conducted after prolonged night phases, showed higher contribution of recently assimilated C (14C) to the root-derived CO2 efflux by shaded plants. Results from 13C natural abundance showed that the cultivation of maize on Chromic Luvisol decreased soil organic matter (SOM) mineralization compared to unplanted soil (negative priming effect). A more important finding is the observed tight coupling of the negative rhizosphere effect on SOM decomposition with photosynthesis.
Similar content being viewed by others
References
Baldocchi D D, Verma S B, Matt D R and Anderson D E 1986 Eddy-correlation measurements of carbon dioxide efflux from the floor of a deciduous forest. J. Appl. Ecol. 23, 967–975.
Biddulph O 1969 Mechanisms of translocation of plant methabol-ites. In Physiological Aspects of Crop Yield. Eds. J D Eastin, F A Haskins, C Y Sullivan, C H M Van Bavel. American Society of Agronomy, Madison, pp. 143–164.
Billes G, Bottner P and Gandais-Riollet N 1988 Effet des racines de graminées sur la minéralisation nette de l'azote du sol. Rev. Ecol. Biol. Sol. 25, 261–277.
Bottner P, Sallih Z and Billes G 1988 Root activity and carbon metabolism in soils. Biol. Fert. Soils 7, 71–78.
Bottner P, Cortez J and Sallih Z 1991 Effect of living roots on carbon and nitrogen of the soil microbial biomass. InBritish Ecolo-gical Society Special Publication 10, Ed. D. Atkinson. Blackwell Scientific Publications, Oxford. pp. 201–210.
Cheng W1996 Measurement of rhizosphere respiration and organic matter decomposition using natural 13 C. Plant Soil 183, 263–268.
Cheng W and Coleman D C 1990 Effect of living roots on soil organic matter decomposition. Soil Biol. Biochem. 22, 781–787.
Cheng W and Kuzyakov Y 2004 Root effects on decomposition of organic matter. In Roots and Soil Management: Interactions Between Roots and Soil. Ed. S Wright. Soil Science Society of America Book Series, Soil Science Society of America, Inc., Madison, Wisconsin, USA. (in press).
Cheng W, Coleman D C, Carroll C R and Hoffman C A 1993 In situ measurement of root respiration and soluble C concentrations in the rhizosphere. Soil Biol. Biochem. 25, 1189–1196.
Cheng W, Coleman D C, Carroll C R and Hoffman C A 1994 Invest-igating short-term carbon flows in the rhizospheres of different plant species, using isotopic trapping. Agron. J. 86, 782–788.
Craine J M, Wedin D A and Chapin F S 1999 Predominance of eco-physiological controls on soil CO 2 flux in a Minnesota grassland. Plant Soil 207, 77–86
Ekblad A, Nyberg G and Högberg P 2002 13 C-discrimination dur-ing microbial respiration of addede C 3-, C 4-and 13 C-labelled sugars to a C 3-forest soil. Oecologia 131, 245–249.
Fu S and Cheng W2002 Rhizosphere priming effects on the decom-position of soil organic matter in C 4 and C 3 grassland soils. Plant Soil 238, 289–294.
Gregory P J and Atwell B J 1991 The fate of carbon in pulse labelled crops of barley and wheat. Plant Soil 136, 205–213.
Helal H M and Sauerbeck D 1986 Effect of plant roots on car-bon metabolism of soil microbial biomass. Z. Pflanzenernährung Bodenk. 149, 181–188.
Helal H M and Sauerbeck D R 1987 Direct and indirect influences of plant roots on organic matter and phosphorus turnover in soil. INTECOL Bull. 15, 49–58.
Helal H M and Sauerbeck D 1989 Carbon turnover in the rhizo-sphere. Z. Pflanzenernährung Bodenk. 152, 211–216.
Hodge A, Paterson E, Thornton B, Millard P and Killham K 1997 Effects of photon flux density on carbon partitioning and rhizosphere carbon flow of Lolium perenne. J. Exp. Bot. 48, 1797–1805.
Högberg P, Nordgren A, Buchmann N, Taylor A F S, Ekblad A, Ho-gberg MN, Nyberg G, Ottosson-Lofvenius Mand Read D J 2001 Large-scale forest girdling shows that current photosynthesis drives soil respiration. Nature 411: 6839, 789–792.
Jenkinson D S, Fox R H and Rayner J H 1985 Interactions between fertilizer nitrogen and soil nitrogen–the so-called 'priming' effect. J. Soil Sci. 36, 425–444.
Johnen B G and Sauerbeck D 1977 A tracer technique for measuring growth, mass and microbial breakdown of plant roots during vegetation. In Soil Organisms as Components of Ecosystems. Eds. U Lohm, T Persson. pp. 366–373. Ecological Bulletins, Stockholm, 25, 366–373.
Kim J and Verma S B 1992 Soil surface CO 2 flux in a Minnesota peatland. Biogeochemistry 18, 37–51.
Kuzyakov Y 2002 Review: Factors affecting rhizosphere priming effects. J. Plant Nutr. Soil Sci. 165, 382–396.
Kuzyakov Y and Domanski G 2000 Carbon input by plants into the soil. Review. Z. Pflanzenernährung Bodenk. 163, 421–431.
Kuzyakov Y and Cheng W 2001 Photosynthesis controls of rhizo-sphere respiration and organic matter decomposition. Soil Biol. Biochem. 33, 1915–1925.
Kuzyakov Y, Kretzschmar A and Stahr K 1999 Contribution of Lolium perenne rhizodeposition to carbon turnover of pasture soil. Plant Soil 213, 127–136.
Kuzyakov Y, Friedel J K and Stahr K 2000 Review of mechanisms and quantification of priming effects. Soil Biol. Biochem. 32, 1485–1498.
Kuzyakov Y, Ehrensberger H and Stahr K 2001 Carbon partitioning and below-ground translocation by Lolium perenne. Soil Biol. and Biochem 33, 61–74.
Kuzyakov Y, Leinweber P, Sapronov D, Eckhardt K-U 2003 Qual-itative assessment of root exudates in non-sterilized soil by analytical pyrolysis. J. Plant Nutr. Soil Sci. 166, 719–723.
Lin G and Ehleringer J R 1997 Carbon isotopic fractionation does not occur during dark respiration in C 3 and C 4 plants. Plant Physiol. 114, 391–394.
Mary B, Fresneau C, Morel J L and Mariotti A 1993 C and N cycling during decomposition of root mucilage, roots and glucose in soil. Soil Biol. Biochem. 25, 1005–1014.
Martin J K and Merckx R 1992 The partioning of photosynthetically fixed carbon within the rhizosphere of mature wheat. Soil Biol. Biochem 24, 1147–1156.
Meharg A A 1994 A critical review of labelling techniques used to quantify rhizosphere carbon–flow. Plant Soil 166, 55–62.
Meharg A A and Killham K 1990 Carbon distribution within the plant and rhizosphere in laboratory and field grown Lolium per-enne at different stages of development. Soil Biol Biochem. 22, 471–477.
Nguyen C, Todorovic C, Robin C, Christophe A and Guckert A 1999 Continuous monitoring of rhizosphere respiration after labelling of plant shoots with 14 CO 2. Plant Soil 212, 191–201.
Oberbauer S F, Gillespie C T, Cheng W, Sala A, Gebauer R and Tenhunen J D 1996 Diurnal and seasonal patterns of ecosystem CO 2 efflux from upland tundra in the foothills of the Brooks Range, Alaska, U.S.A. Arct. Alp. Res. 28, 328–338.
Qian J H, Doran J W and Walters D T 1997 Maize plant contribu-tions to root zone available carbon and microbial transformations of nitrogen. Soil Biol. Biochem 29, 1451–1462.
Rochette P and Flanagan L B 1998 Quantifying rhizosphere respir-ation in a corn crop under field conditions. Soil Sci Soc Amer J. 61, 466–474.
Raich J W and Schlesinger W H 1992 The global carbon diox-ide flux in soil respiration and its relationship to vegetation and climate. Tellus 44, 81–99.
Reid J B and Goss M J 1982 Suppression of decomposition of 14 C carbon isotope-labelled plant roots in the presence of living roots of maize and perennial ryegrass. J. Soil Sci. 33, 387–395.
Reid J B and Goss M J 1983 Growing crops and transformations of 14 C-labelled soil organic matter. Soil Biol. Biochem. 15, 687–691.
Saggar S, Hedley C and Mackay AD 1997 Partitioning and trans-location of photosyntetically fixed 14 C in grazed hill pastures. Biol. Fert Soils 25, 152–158.
Sallih Z and Bottner P 1988 Effect of wheat (Triticum aestivum) roots on mineralization rates of soil organic matter. Biol. Fert. Soils 7, 67–70.
Sparling G P, Cheshire M V and Mundie C M 1982 Effect of barley plants on the decomposition of 14 C-labelled soil organic matter. J. Soil Sci. 33, 89–100.
Swinnen J, Van Veen J A and Merckx R 1994 14 C pulse–labelling of field–grown spring wheat: an evaluation of its use in rhizosphere carbon budget estimations. Soil Biol. Biochem. 26, 161–170.
Swinnen J, Van Veen J A and Merckx R 1995 Root decay and turnover of rhizodeposits estimated by 14 C pulse-labelling in field-grown winter wheat and spring barley. Soil Biol. Biochem. 27, 211–217.
Todorovic C, Nguyen C, Robin C and Guckert A 2001 Root and mi-crobial involvement in the kinetics of 14 C-partitioning to rhizo-sphere respiration after a pulse labelling of maize assimilates. Plant Soil 228, 179–189.
Warembourg F R and Billes G 1979 Estimating carbon transfers in the plant rhizosphere. In The Soil-Root Interface. Eds. J L Harley and R Scott Russell. pp. 183–196. Academic Press, London.
Warembourg F R and Paul E A 1977 Seasonal transfers of assim-ilated s14 C in grassland: plant production and turnover, soil and plant respiration. Soil Biol. Biochem. 9, 295–301.
Whipps J M 1987 Carbon loss from the roots of tomato and pea seedlings grown in soil. Plant Soil 103, 95–100.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kuzyakov, Y., Cheng, W. Photosynthesis controls of CO2 efflux from maize rhizosphere. Plant and Soil 263, 85–99 (2004). https://doi.org/10.1023/B:PLSO.0000047728.61591.fd
Issue Date:
DOI: https://doi.org/10.1023/B:PLSO.0000047728.61591.fd