Abstract
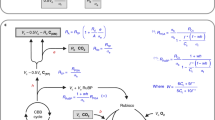
Carbon isotope discrimination during photosynthetic CO2 assimilation has been extensively studied and rigorous models have been developed, while the fractionations during photorespiratory and dark respiratory processes have been less well investigated. Whilst models of discrimination have included specific factors for fractionation during respiration (e) and photorespiration (f), these effects have been considered to be very small, i.e. not significantly modifying the net discrimination expressed in organic material. On this paper we consider the fractionation effects associated with specific reactions set against the overall discrimination which occurs during source-product transformations. We review the studies which have recently shown that discrimination occurs during respiration at night in intact C3 leaves, leading to the production of CO2 enriched in 13C (i.e., e = −6‰ ), and modifying the signature of the remaining plant material. Under photorespiratory conditions (i.e. increased oxygen concentration and high temperature), the photorespiratory fractionation factor may be high (with f around +10‰ ), and significantly alters the observed net photosynthetic discrimination measured during gas exchange. Fractionation factors for both respiration and photorespiration have been shown to be variable among species and with environmental conditions, and we suggest that the term `apparent fractionation' be used to describe the net effect for each process. In this paper we review the fractionations during photorespiration and dark respiration and the metabolic origin of the CO2 released during these processes, and we discuss the ecological implications of such fractionations.
Similar content being viewed by others
References
Abelson H & Hoering TC (1961) Carbon isotope fractionation in formation of aminoacids by photosynthetic organisms. PNAS 47: 623–632.
Atkin OK, Evans JR & Siebke K (1998) Relationship between the inhibition of leaf respiration by light and enhancement of leaf dark respiration following light treatment. Aust. J. Plant. Physiol. 25: 437–443
Atkin OK, Evans JR, Ball MC, Lambers H & Pons TL (2000) Leaf respiration of snow gum in the light and dark. Interactions between temperature and irradiance. Plant Physiol. 122: 915–924.
Baertschi P (1953) Die Fraktionierung der natürlichen Kohlenstoffisotopen im Kohlendioxydstoffwechsel grüner Planzen. Helvetica Chim. Acta 95: 773–781.
Blair N, Leu A, Munoz E, Olsen J, Kwong E & Desmarais D (1985) Carbon isotopic fractionation in heterotrophic microbial metabolism. App. Env. Microbiol. 50 (4): 996–1001.
Bort J, Brown RH & Araus JL (1996) Refixation of respiratory CO2 in the ears of C-3 cereals. J. Exp. Bot. 47: 1567–1575.
Brooks A & Farquhar GD (1985) Effect of temperature on the CO2/O2 specificity of ribulose-1,5-bisphosphate carboxylase/oxygenase and the rate of respiration in light. Planta 165: 397–406.
Brooks JR, Flanagan LB, Varney GT & Ehleringer JR (1997) Vertical gradients in photosynthetic gas exchange characteristics and refixation of respired CO2 within boreal forest canopies. Tree Physiol. 17: 1–12.
Bowling DR, McDowell NG, Bond BJ, Law BE & Ehleringer JR (2002) C-13 content of ecosystem respiration is linked to precipitation and vapor pressure deficit. Oecologia 131: 113–124.
Bowling DR, Tans PP & Monson RK (2001) Partitioning net ecosystem carbon exchange with isotopic fluxes of CO2. Global Change Biol. 7: 127–145.
Brugnoli E, Hubick KT, von Caemmerer S, Wong SC & Farquhar GD (1988) Correlation between the carbon isotope discrimination in leaf starch and sugars of C3 plants and the ratio of intercellular and atmospheric partial pressures of carbon dioxide. Plant Physiol. 88: 1418–1424.
Brugnoli E & Farquhar GD (2000) Photosynthetic fractionation of carbon isotopes. In: Leegood RC, Sharkey TD & von Caemmerer (eds) Photosynthesis: Physiology and Metabolism (pp. 399–434). Kluwer Academic Publishers, the Netherlands.
Buchmann N, Brooks JR, Flanagan LB & Ehleringer JR (1998)Carbon isotope discrimination of terrestrial ecosystems. In: Griffiths H (ed) Stable Isotopes and the Integration of Biological, Ecological and Geochemical Cycles (pp. 203–221). BIOS Scientific, Oxford.
Buchmann N, Kao WY & Ehleringer J (1997) Influence of stand structure on carbon-13 of vegetation, soils, and canopy air within deciduous and evergreen forests in Utah, United States. Oecologia 110: 109–119.
Ciais P & Meijer HAL (1998) The 18O/16O isotope ratio of atmospheric CO2 and its role in global carbon cycle research ecosystems. In: Griffiths H (ed) Stable Isotopes: Integration of Biological, Ecological and Geochemical Processes (pp. 409–431). Environmental plant biology series, Bios Scientific Publishers, Oxford, UK.
Ciais P, Friedlingstein P, Schimel DS & Tans PP (1999) A global calculation of the delta C-13 of soil respired carbon: Implications for the biospheric uptake of anthropogenic CO2. Global Biogeochem. 13: 519–530.
Ciais P, Tans PP, White JWC, Trolier M, Francey RJ, Berry JA, Randall DR, Sellers PJ, Collatz JG & Schimel DS (1995a) Partitioning of Ocean and Land Uptake of CO2 as Inferred by Delta-C-13 Measurements from the Noaa Climate Monitoring and Diagnostics Laboratory Global Air Sampling Network. J. Geophys. Res.-Atmos. 100: 5051–5070.
Ciais P, Tans PP, Trolier M, White JWC & Francey RJ (1995b) A Large Northern-Hemisphere Terrestrial CO2 Sink Indicated by the C-13/C-12 Ratio of Atmospheric CO2. Science 269: 1098–1102.
Craig H (1954) Carbon 13 in plants and the relationship between carbon 13 and carbon 14 variation in nature. J. Geol. 62: 115–149.
Damesin C & Lelarge C (2003) Carbon isotope composition of current-year shoots from Fagus sylvatica in relation to growth, respiration and use of reserves. Plant Cell Environ. 26: 207–219.
Deléens E, Schwebel-Dugué N & Trémolières A (1984) Carbon isotope composition of lipidic classes isolated from tobacco leaves. FEBS Lett. 178: 55–58.
DeNiro MJ & Epstein S (1977) Mechanism of carbon isotope fractionation associated with lipid synthesis. Science 197: 261–263.
Duranceau M, Ghashghaie J, Badeck F, Deleens E & Cornic G (1999) δ13C of CO2 respired in the dark in relation to δ13C of leaf carbohydrates in Phaseolus vulgaris L. under progressive drought. Plant Cell Environ. 22: 515–523.
Duranceau M, Ghashghaie J & Brugnoli E (2001) Carbon Isotope discrimination during photosynthesis and dark respiration in intact leaves of Nicotiana sylvestris: comparison between wild type and mitochondrial mutant plants. Aust. J. Plant. Physiol. 28(1): 65–71.
Ekblad A & Hogberg P (2000) Analysis of delta C-13 of CO2 distinguishes between microbial respiration of added C-4-sucrose and other soil respiration in a C-3-ecosystem. Plant and Soil 219: 197–209.
Ekblad A & Hogberg P (2001) Natural abundance of C-13 in CO2 respired from forest soils reveals speed of link between tree photosynthesis and root respiration. Oecologia 127: 305–308.
Ekblad A, Nyberg G & Hogberg P (2002) C-13-discrimination during microbial respiration of added C-3-, C-4-and C-13-labelled sugars to a C-3-forest soil. Oecologia 131: 245–249.
Evans JR, Sharkey TD, Berry JA & Farquhar GD (1986) Carbon isotope discrimination measured concurrently with gas exchange to investigate CO2 diffusion in leaves of higher plants. Austral. J. Plant Physiol. 13: 281–292.
Evans & Loreto (2000) Acquisition and diffusion of CO2 in higher plant leaves. In: Leegood RC, Sharkey TD & von Caemmerer (eds) Photosynthesis: Physiology and Metabolism (pp. 321–351). Kluwer Academic Publishers, the Netherlands.
Farquhar GD (1980) Carbon isotope discrimination by plants and the ratio of intercellular and atmospheric CO2 concentrations. In: Pearman GI (ed) Carbon Dioxide and Climate: Australian Research (pp. 105–110). Australian Academy of Science, Canberra.
Farquhar GD, O'Leary MH & Berry JA (1982) On the relationship between carbon isotope discrimination and the intercellular carbon dioxide concentration in leaves. Austr. J. Plant Physiol. 9: 121–137.
Farquhar GD, Hubick KT, Condon AG & Richards RA (1989) Carbon isotope fractionation and water-use efficiency. In: Rundel PW, Ehleringer JR & Nagy KA (eds), Satble Isotopes in Ecological Research. Ecological Studies, Vol. 68 (pp. 21–40). Springer-Verlag, New York.
Gerbaud A & Andre M (1987) An evaluation of the recycling in measurements of photorespiration. Plant Physiol. 83: 933–937.
Ghashghaie J, Duranceau M, Badeck F, Cornic G, Adeline MT & Deleens E (2001) δ13C of CO2 respired in the dark in relation to leaf metabolites: comparisons between Nicotiana sylvestris and Helianthus annuus under drought. Plant Cell Environ. 24: 505–515.
Gillon JS (1997) Carbon isotope discrimination: interactions between respiration, leaf conductance and photosynthetic capacity. Ph.D. Thesis. University of Newcastle upon Tyne, Newcastle.
Gillon JS & Griffiths H (1997) The influence of (photo)respiration on carbon isotope discrimination in plants. Plant Cell Environ. 20: 1217–1230.
Gillon JS, Borland AM, Harwood KG, Roberts A, Broadmeadow MSJ & Griffiths H (1998) Carbon isotope discrimination in terrestrial plants: carboxylations and decarboxylations. In: Griffiths H (ed) Stable Isotopes: Integration of Biological, Ecological and Geochemical Processes (pp. 203–221). Environmental plant biology series, Bios scientific publishers, Oxford, UK.
Gleixner G, Danier HJ, Werner RA & Schmidt HL (1993) Correlation between the 13C content of primary and secondary plant products in different cell compartments and that in decomposing basidiomycetes. Plant. Physiol. 102: 1287–1290.
Gleixner G & Schmidt HL (1997) Carbon isotope effects on the fructose-1,6-bisphosphate aldolase reaction, origin for nonstatistical 13C distribution in carbohydrates. J. Biol. Chem. 272(9): 5382–5387.
Gleixner G, Scrimgeour C, Schmidt HL & Viola R (1998) Stable Isotope distribution in the major metabolites of source and sink organs of Solanum tuberosum L.: A powerful tool in study of metabolic partitioning in intact plants. Planta 207: 241–245.
Griffiths H, Borland A, Gillon JS, Harwood K, Maxwell K & Wilson J (1999) Stable isotopes reveal exchanges between soil, plants and the atmosphere. In: Press MC, Scholes JD & Barker MG (eds) Physiological Plant Ecology. Blackwell Science.
Guy RD, Fogel ML & Berry JA (1993) Photosynthetic Fractionation of the Stable Isotopes of Oxygen and Carbon. Plant Physiol. 101: 37–47.
Harwood KG, Gillon JS, Griffiths H & Broadmeadow MSJ (1998) Diurnal variation of CO2δ13C and δ18O and evaporative site enrichment of H2O δ18O in Piper aduncum under field conditions in Trinidad. Plant Cell Environ. 21: 269–283.
Henderson SA, von Caemmerer S & Farquhar GD (1992) Shortterm measurements of carbon isotope discrimination in several C4 species. Aust. J. Plant Physiol. 19: 263–285.
Hill SA & Bryce JH (1992) Malate metabolism and light-enhanced dark respiration in barley mesophyll protoplasts. In: Lambers H, van der Plas LHW (eds) Molecular, Biochemical and Physiological Aspects of Plant Respiration (pp. 221–230). SPB Academic Publishing, The Hague, The Netherlands.
Hsu JC & Smith BN (1972) 13C/12C ratios of carbon dioxide from peanut and sunflower seedlings and tobacco leaves in light and in darkness. Plant Cell Physiol. 13: 689–694
Igamberdiev AU, Ivlev AA, Bykova NV, Threlkeld CN, Lea PJ & Gardestrom P (2001) Decarboxylation of glycine contributes to carbon isotope fractionation in photosynthetic organisms. Photosynth. Res. 67: 177–184.
Ivlev AA, Bykova NV & Igamberdiev AU (1996) Fractionation of carbon (C-13/C-12) isotopes in glycine decarboxylase reaction. FEBS Letts. 386: 174–176.
Ivlev AA, Igamberdiev AU, Threlkeld C & Bykova NV (1999) Carbon isotope effects in the glycine decarboxylase reaction in vitro on mitochondria from pea and spinach. Russ. J. Plant Physiol. 46: 653–660.
Ivlev AA (2001) Carbon isotope effects (C-13/C-12) in biological systems. Separ. Sci. Tech. 36: 1819–1914.
Ivlev AA (2002) Carbon isotope (C-13/C-12) effect of photorespiration in photosynthesizing organisms. Evidence in favor of the existence and a possible mechanism. Biofizika 47: 55–70.
James WO (1953) Plant Respiration. Oxford University Press, Amen House, London.
Jordan F, Kuo DJ, & Monse EU (1978) Carbon kinetic isotope effects on pyruvate decarboxylation catalysed by yeast pyruvate decarboxylase and models. J. Amer. Chem. Soc. 100 (9): 2872–2878.
Jacobson BS, Smith BN, Epstein S & Laties GG (1970) The prevalence of carbon 13 in respiratory carbon dioxide as an indicator of the type of endogenous substrate: The change from lipid to carbohydrate during the respiratory rise in potato slices. J. Gen. Physiol. 55: 1–17.
Keeling CD (1958) The concentration and isotopic abundances of carbon dioxide in rural areas. Geochim. Cosmochim. Acta 13: 322–334.
Lin G & Ehleringer JR (1997) Carbon isotopic fractionation does not occur during dark respiration of C3 and C4 plants. Plant Physiol. 114: 191–194.
Lloyd J & Farquhar GD (1994) C-13 Discrimination During CO2 Assimilation by the Terrestrial Biosphere. Oecologia 99: 201–215.
Loreto F, Velikova V & Di Marco G (2001) Respiration in the light measured by (CO2 )-C-12 emission in (CO2 )-C-13 atmosphere in maize leaves. Aus. J. Plant Physiol. 28: 1103–1108.
Melzer E & Schmidt HL (1987) Carbon isotope effects on the pyruvate dehydrogenase reaction and their importance for relative carbon-13 depletion in lipids. J. Biol. Chem. 262: 8159–8164.
O'Leary MH (1980) Determination of heavy-atom isotope effects on enzyme-catalyzed reactions. Meth. Enzymol. 64: 83–107.
O'Leary MH (1981) Carbon Isotope Fractionation in plants. Phytochemistry 20: 553–567.
O'Leary MH, Madhavan S & Paneth P (1992) Physical and chemical basis of carbon isotope fractionation in plants. Plant Cell Environ. 15: 1099–1104.
Park R & Epstein S (1961) Metabolic fractionation of 13C and 12C in plants. Plant Physiol. 36: 133–138.
Parnik T, Keerberg O & Viil J (1972) Decarboxylation of primary and end-products of photosynthesis at different oxygen concentrations. J. Exp. Bot. 46: 1439–1447.
Raven JA, Griffiths H, Glidewell SM & Preston T (1982) The Mechanism of Oxalate Biosynthesis in Higher-Plants – Investigations with the Stable Isotopes O-18 and C-13. Proc. Royal Soc. B. 216: 87–101.
Rooney MA (1988) Short-term carbon isotope fractionation by plants. Ph.D Thesis. University of Wisconsin, Wisconsin.
Rossmann A, Butzenlechner M & Schmidt HL (1991) Evidence for a non-statistical carbon isotope distribution in natural glucose. Plant Physiol. 96: 609–614.
Rossmann A, Schmidt HL, Reniero F, Versini G, Moussa I & Merle MH (1996) Stable carbon isotope content in ethanol of EC data bank wines from Italy, France and Germany. Zeitschr. Lebensmittel-Untersuch. Forsch. 203: 293–301.
Schmidt HL & Gleixner G (1998) Carbon isotope effects on key reactions in plant metabolism and 13C-patterns in natural compounds. In: Griffiths H (ed) Stable Isotopes: Integration of biological, ecological and geochemical processes (pp. 13–25). Environmental plant biology series. Bios Scientific Publishers, Oxford, UK.
Smith BN (1971) Carbon isotope ratios of respired CO2 from castor bean, peanut, pea, radish, squash, sunflower and wheat seedlings. Plant Cell Physiol. 12: 451–455.
Tcherkez G, Nogués S, Bleton J, Cornic G., Badeck F.-W & Ghashghaie J (2003) Metabolic origin of carbon isotope composition of leaf dark-respired CO2 in Phaseolus vulgaris L. Plant Physiol. 131: 237–244.
Troughton JH, Card KA, & Hendy CH (1974) Photosynthetic pathways and carbon isotope discrimination by plants. Carnegie Inst. Washington Yearb. 73: 768–780.
Vogel JC (1980) Fractionation of the carbon isotopes during photosynthesis. In: Sitzungsberichte der Heidelberger Akademie der Wissenschaften, matematisch-naturwissen-schaftlichs Klasse Jahrgang, 3 Abhandlung (pp. 111–135). Springer-Verlag, Berlin/ New York.
von Caemmerer S & Evans JR (1991) Determination of the average partial pressure of CO2 in chloroplasts from leaves of several C3 plants. Aust. J. Plant Physiol. 18: 287–305.
Yakir D & Wang XF (1996) Fluxes of CO2 and water between terrestrial vegetation and the atmosphere estimated from isotope measurements. Nature 380: 515–517.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ghashghaie, J., Badeck, FW., Lanigan, G. et al. Carbon isotope fractionation during dark respiration and photorespiration in C3 plants. Phytochemistry Reviews 2, 145–161 (2003). https://doi.org/10.1023/B:PHYT.0000004326.00711.ca
Issue Date:
DOI: https://doi.org/10.1023/B:PHYT.0000004326.00711.ca