Abstract
Purpose. The aim of the present work is to study the interaction of phosphate salts with trehalose and sucrose in freeze-dried matrices, particularly the effect of the salts on the glass transition temperature (Tg) of the sugars.
Methods. Freeze-dried trehalose and sucrose systems containing different amounts of sodium or potassium phosphate were analyzed by differential scanning calorimetry to determine the Tg and by Fourier-transform infrared spectroscopy (FTIR) analysis to evaluate the strength of the interaction between sugars and phosphate ions.
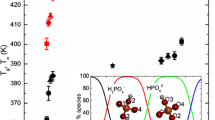
Results. Sucrose-phosphate mixtures show an increase in Tg up to 40°C in a broad pH range (4-9) compared to that of pure sucrose. Sucrose-phosphate mixtures exhibit a higher Tg than pure sucrose while retaining higher water contents. Trehalose-phosphate mixtures (having a Tg of 135°C at a pH of 8.8) are a better option than pure trehalose for preservation of labile materials. The -OH stretching of the sugars in the presence of phosphates decreases with increase in pH, indicating an increase in the sugar-phosphate interaction.
Conclusions. Sugar-phosphate mixtures exhibit several interesting features that make them useful for lyophilization of labile molecules; Tg values much higher than those observed for the pure sugars can be obtained upon the addition of phosphate.
Similar content being viewed by others
REFERENCES
S. Duddu and P. DalMonte. Effect of glass transition temperature on the stability of lyophilized formulations containing a chimeric therapeutic monoclonal antibody. Pharm. Res. 14:591 (1997).
J. Buitink, J. van den Dries, F. Hoekstra, M. Alberda, and M. Hemminga. High critical temperature above Tg may contribute to the stability of biological systems. Biophys. J. 79:1119-1128 (2000).
M. Uritani, M. Takai, and K. Yoshinaga. Protective Effect of Disaccharides On Restriction Endonucleases During Drying Under Vacuum. J. Biochem. 117:774 (1995).
J. H. Crowe, J. F. Carpenter, and L. M. Crowe. The role of vitrification in anhydrobiosis. Annu. Rev. Physiol. 6:73-103 (1998).
L. M. Crowe, D. Reid, and J. H. Crowe. Is trehalose special for preserving dry biomaterials? Biophys. J. 71:2087-2093 (1996).
S. Tzannis and S. Prestrelski. Moisture effects on proteinexcipient interactions in spray-dried powders. Nature of destabilizing effects of sucrose. J. Pharm. Sci. 88:360 (1999).
T. W. Randolph. Phase separation of excipients during lyophilization: Effects on protein stability. J. Pharm. Sci. 86:1198-1203 (1997).
K. Koster and A. C. Leopold. Sugars and desiccation tolerance in seeds. Plant Physiol. 88:829-832 (1988).
J. Carpenter, S. Hand, L. Crowe, and J. Crowe. Cryoprotection of Phosphofructokinase with Organic Solutes—Characterization of Enhanced Protection in the Presence of Divalent-Cations. Arch. Biochem. Biophys. 250:505-512 (1986).
N. Morel-Desrosier, C. Lhermet, and J. P. Morel. Interactions between cations and sugars. Part 6. Calorimetric method for simultaneous determination of the stability constant and enthalpy change for weak complexation. J. Chem. Soc. Faraday T 87:2173-2177 (1991).
P. Rongere, N. Morel-Desrosiers, and J. Morel. Interactions Between Cations and Sugars. 8. Gibbs Energies, Enthalpies and Entropies of Association of Divalent and Trivalent Metal-Cations with Xylitol and Glucitol in Water at 298.15 K. J. Chem. Soc. Faraday T. 91:2771-2777 (1995).
D. P. Miller, J. J. de Pablo, and H. R. Corti. Thermophysical properties of trehalose and its concentrated aqueous solutions. Pharm. Res. 14:578-590 (1997).
D. P. Miller, R. E. Anderson, and J. J. de Pablo. Stabilization of lactate dehydrogenase following freeze-thawing and vacuum drying in the presence of trehalose and borate. Pharm. Res. 15:1215-1221 (1998).
D. P. Miller, J. J. de Pablo, and H. R. Corti. Viscosity and glass transition temperature of aqueous mixtures of trehalose with borax and sodium chloride. J. Phys. Chem. B 103:10243-10249 (1999).
P. B. Conrad, D. P. Miller, P. R. Cielenski, and J. J. de Pablo. Stabilization and preservation of Lactobacillus acidophilus in saccharide matrices. Cryobiology 41:17-24 (2000).
P. Conrad. Preservation of biological molecules and living cells. Ph D Thesis. (2000).
N. Ekdawi-Sever, P. B. Conrad, and J. J. de Pablo. The effects of annealing on freeze-dried Lactobacillus acidophilus. J. Food Sci. 68:2504-2511 (2003).
S. Ohtake, C. Schebor, S. P. Palecek, and J. J. de Pablo. Effect of sugar-phosphate mixtures on the stability of DPPC membranes in dehydrates systems. Cryobiology 48:81-89 (2004).
L. Greenspan. Humidity fixed points of binary saturated aqueous solutions. J. Res. 81:89-96 (1977).
W. F. Wolkers, H. Oldenhof, M. Alberda, and F. A. Hoekstra. A Fourier transform infrared microspectroscopy study of sugar glasses: application to anhydrobiotic higher plant cells. Biochim. Biophys. Acta 1379:83-96 (1998).
M. F. Mazzobre, M. P. Longinotti, and H. R. Corti. Effect of salts on the properties of aqueous sugar systems, in relation to biomaterial stabilization. 1. Water sorption behaviour and ice crystallization/melting. Cryobiology 43:199-210 (2001).
L. S. Taylor and G. Zografi. Sugar-polymer hydrogen bond interactions in lyophilized amorphous mixtures. J. Pharm. Sci. 87:1615-1621 (1998).
L. van den Berg and D. Rose. Effect of freezing on the pH and composition of sodium and potassium phosphate solutions: the reciprocal system KH2PO4-Na2HPO4-H2O. Arch. Biochem. Biophys. 81:319-329 (1959).
D. R. MacFarlane, J. Pringle, and G. Annat. Reversible selfpolymerizing high Tg lyoprotectants. Cryobiology 45:188-192 (2002).
N. Murase, P. Echlin, and F. Franks. The structural states of freeze-concentrated and freeze-dried phosphates studied by scanning electron microscopy and differential scanning calorimetry. Cryobiology 28:364-375 (1991).
M. P. Longinotti, M. F. Mazzobre, and M. P. Buera. Effect of salts on properties of aqueous sugar systems in relation to biomaterial stabilization. 2. Sugar crystallization rate and electrical conductivity behavior. Phys. Chem. Chem. Phys. 4:533-540 (2002).
M. F. Mazzobre and M. P. Buera. Combined effects of trehalose and cations on thermal resistance of B-galactosidase in freeze-dried systems. Biochim. Biophys. Acta 1473:337-344 (1999).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ohtake, S., Schebor, C., Palecek, S.P. et al. Effect of pH, Counter Ion, and Phosphate Concentration on the Glass Transition Temperature of Freeze-Dried Sugar-Phosphate Mixtures. Pharm Res 21, 1615–1621 (2004). https://doi.org/10.1023/B:PHAM.0000041456.19377.87
Issue Date:
DOI: https://doi.org/10.1023/B:PHAM.0000041456.19377.87