Abstract
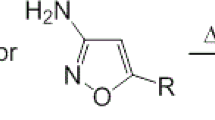
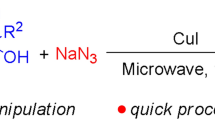
Herein we describe the conversion of a known [3+2] cycloaddition reaction between an azide and an acetylene from a thermally promoted reaction to a microwave assisted process. Modification of conditions including concentration, temperature, solvent type and time were investigated. This methodology study led us to use high concentration and high temperatures to achieve the desired fast reaction times and high yields.
Similar content being viewed by others
References
Gedye, R., Smith, F., Westaway, K., Ali, H., Baldisera, L., Laberge, L. and Rousell, J., The use of microwave ovens for rapid organic synthesis, Tetrahedron Lett., 27 (1986) 279-282.
Giguere, R. J., Bray, T. L., Duncan, S. M. and Majetich, G., Application of commercial microwave ovens to organic synthesis, Tetrahedron Lett., 27 (1986) 4945–4948.
The material described in this paper was previously disclosed at the 1st International Microwaves in Chemistry Conference, 7–9 March 2003, in Gainesville, Florida.
Dalvie, D. K., Kalgutkar, A. S., Khojasteh-Bakht, S. C., Obach, R. S. and O'Donnell, J. P., Biotransformation reactions of five-membered aromatic heterocyclic rings, Chem. Res. Toxicol., 15 (2002) 269–299.
Butler, R. N. and Garvin, V. C., Proton and carbon-13 nuclear magnetic resonance study of the tautomeric composition of the nitrogen analog of nicotine acid, 5-(3-pyridil)tetrazole: A new tetrazole ylide, J. Chem. Res., (S) (1982) 122–123.
Moltzen, E. K., Pedersen, H., Boegesoe, K. P., Meier, E., Frederiksen, K., Sanchez, C. and Lembol, H. L., Biososteres of arecoline: 1,2,3,6-tetrahydro-5-pyridil-substituted and 3-piperidyl-substituted derivatives of tetrazoles and 1,2,3-triazoles. Synthesis and muscarinic activity, J. Med. Chem., 37 (1994) 4085–4099.
Chakrabarti, J. K., Hotten, T. M., Pullar, I. A. and Steggles, D. J., Heteroarenobenzodiazepines. 6. Synthesis and pharmacological evaluation of CNS activities of [1,2,3]triazolo[4,5-b][1,5]-, imidazolo[4,5-b][1,5]-, and pyrido[2,3-b][1,5]benzodiazepines. 10-Piperazinyl-4H-1,2,3-triazolo[4,5-b][1,5]benzodiazepines with neuroleptic activity, J. Med. Chem., 32 (1989) 2375–2381.
Buckle, D. R., Rockell, C. J. M., Smith, H. and Spicer, B. A., Studies on 1,2,3-triazoles. 13. (Piperazinylalkoxy)-[1]benzopyranol[2,3-d]-1,2,3-triazol-9(1H)-ones with combined H1-antihistamine and mast cell stabilizing properties, J. Med. Chem., 29 (1986) 2262–2267.
Romeiro, G. A., Pereira, L. O. R., de Souza, M. C. B. V., Ferreira, V. F. and Cunha, A. C., A new and efficient procedure for preparing 1,2,3-triazoles, Tetrahedron Lett., 38 (1997) 5103–5106.
Grassivaro, N., Rossi, E. and Stradi, R., Enamines: Part 46. Synthesis of 5-Dialkylamino-1-aryl-1,2,3-triazoles. Functionalized at C-4, Synthesis, Synthesis, (1986) 1010–1012.
Prager, R. H. and Razzino, P., Heterocyclic synthesis with azides. III. Reactions of triazoles made from arylmethylidenemalonates, Aust. J. Chem., 47 (1994) 1375–1385.
Cottrell, I. F., Hands, D., Houghton, P. G., Humphrey, G. R. and Wright, S. H. B., An improved procedure for the preparation of 1-benzyl-1H-1,2,3-triazoles from benzyl azides, J. Heterocyclic. Chem., 28 (1991) 301–304.
For other examples of the use of microwave irradiation to promote azide-alkyne cycloadditions: a)Louerat, F., Bougrin, K., Loupy, A., Retana, A. M. O., Pagalday, J. and Palacios, F., Cycloaddition reactions of azidomethyl phosphonate with acetylenes and enamines. Synthesis of triazoles, Heterocycles, 48 (1998) 161–170.
Katritzky, A. R. and Singh, S. K., Synthesis of Ccarbamoyl-1,2,3-triazoles by microwave induced 1,3-dipolar cycloaddition of organic azides to acetylenic amides, J. Org. Chem. 67, (2002) 9077–9079. For a review of the use of microwave irradiation to promote cycloadditions
de la Hoz, A., Diaz-Ortis, A., Moreno, A. and Langa, F., Cycloaddition under microwave irradiation conditions: Methods and applications, Eur. J. Org. Chem., (2000) 3659–3673.
Abu-Orabi, S. T., Atfah, M. A., Jibril, I., Mari'i, F.M. and Ali, A. A., Dipolar cycloaddition reactions of organic azides with some acetylenic compounds, J. Heterocyclic Chem., 26 (1989) 1461–1468.
Microwave Synthesis: Chemistry at the Speed of Light. Brittany L. Hayes, CEM Publishing, 2002, Matthews, North Carolina.
Microwaves in Organic Synthesis. Andre Loupy (ed.), Wiley-VCH Verlag GMbH & Co. KgaA, Weinheim, 2002.
Saillard, R., Poux, M., Berlan, J. and Audhuy-Peaudecerf, M., Microwave heating of organic solvents: Thermal effects and field modeling, Tetrahedron, 51 (1995) 4033–4042.
Loupy, A., Solvent free reactions, Topics in Current Chemistry, 206 (1999) 155–207.
Bougrin, K. and Soufiaoui, M., Nouvelle voie de synthèse des arylimidazoles sous irradiation micro-ondes en ‘milieu sec’, Tetrahedron Lett., 36 (1995) 3683–3686.
Lidstrom, P., Tierney, J., Wathey, B. and Westman, J., Microwave assisted organic synthesis – A review, Tetrahedron, 57 (2001) 9225–9283.
Caddick, S., Microwave assisted organic reactions, Tetrahedron, 51 (1995) 10403–10432.
Abramovitch, R. A., Applications of microwave energy in organic chemistry. A review, Org. Prep. Proc. Int., 23 (1991) 683–711.
Strauss, C. R. and Trainor, R. W., Developments in microwave-assisted organic chemistry, Aust. J. Chem. 48, (1995) 1665–1692.
Mingos, D. M. P. and Baghurst, D. R., Applications of microwave dielectric heating effects to synthetic problems in chemistry, Chem. Soc. Rev., 20 (1991) 1–47.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Savin, K.A., Robertson, M., Gernert, D. et al. A study of the synthesis of triazoles using microwave irradiation. Mol Divers 7, 171–174 (2003). https://doi.org/10.1023/B:MODI.0000006801.27748.3b
Issue Date:
DOI: https://doi.org/10.1023/B:MODI.0000006801.27748.3b