Abstract
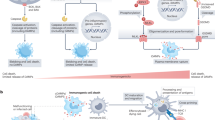
This review summarizes the main aspects and newest findings of how proteinase-activated receptor 1 (PAR-1) may modulate programmed cell death. Activation of PAR-1 has been found to induce or inhibit apoptosis in a variety of cells, depending on the dosage of its physiological agonist thrombin, or that of synthetic receptor activators. To date, cellular targets for PAR-1-mediated effects on apoptosis include neuronal, endothelial, and epithelial cells, fibroblasts, and tumor cells. The signaling pathways involved in the induction or prevention of apoptosis by PAR-1 activation are diverse, and include JAK/STAT, RhoA, myosin light chain kinase, ERK1/2, and various Bcl-2 family members. In view of the well-established involvement of microbial proteinases in host tissue malfunction, the article also elaborates on the possible significance of PAR-1 activation for the pathogenesis of infectious disease.
Similar content being viewed by others
References
Dery O, Corvera CU, Steinhoff M, Bunnett NW. Proteinase-activated receptors: Novel mechanisms of signaling by serine proteases. Am J Physiol 1998; 274: C1429–C1452.
Macfarlane SR, Seatter MJ, Kanke T, Hunter GD, Plevin R. Proteinase-activated receptors. Pharmacol Rev 2001; 53: 245–282.
Vu TK, Hung DT, Wheaton VI, Coughlin SR. Molecular cloning of a functional thrombin receptor reveals a novel proteolytic mechanism of receptor activation. Cell 1991; 64: 1057–1068.
Hollenberg MD, Compton SJ. International Union of Pharmacology. XXVIII. Proteinase-activated receptors. Pharmacol Rev 2002; 54: 203–217.
Vergnolle N, Wallace JL, Bunnett NW, Hollenberg MD. Protease-activated receptors in inflammation, neuronal signaling and pain. Trends Pharmacol Sci 2001; 22: 146–152.
Bizios R, Lai L, Fenton JW, Malik AB. Thrombin-induced chemotaxis and aggregation of neutrophils. J Cell Physiol 1986; 128: 485–490.
Buresi MC, Schleihauf E, Vergnolle N, et al. Protease-activated receptor-1 stimulates Ca(2+)-dependent Cl(−) secretion in human intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol 2001; 281: G323–G332.
Garcia JG, Siflinger-Birnboim A, Bizios R, Del Vecchio PJ, Fenton JW, Malik AB. Thrombin-induced increase in albumin permeability across the endothelium. J Cell Physiol 1986; 128: 96–104.
Vergnolle N. Modulation of visceral pain and inflammation by protease-activated receptors. Br J Pharmacol 2004; 141: 1264–1274.
Gustafson GT, Lerner U. Thrombin, a stimulator of bone resorption. Biosci Rep 1983; 3: 255–261.
Donovan FM, Pike CJ, Cotman CW, Cunningham DD. Thrombin induces apoptosis in cultured neurons and astrocytes via a pathway requiring tyrosine kinase and RhoA activities J Neu-rosci 1997; 17: 5316–5326.
Huang YQ, Li JJ, Karpatkin S. Thrombin inhibits tumor cell growth in association with up-regulation of p21(waf/cip1) and caspases via a p53-independent, STAT-1-dependent pathway. J Biol Chem 2000; 275: 6462–6468.
Zain J, Huang YQ, Feng X, Nierodzik ML, Li JJ, Karpatkin S. Concentration-dependent dual effect of thrombin on impaired growth/apoptosis or mitogenesis in tumor cells. Blood 2000; 95: 3133–3138.
Chin AC, Vergnolle N, MacNaughton WK, Wallace JL, Hollenberg MD, Buret AG. Proteinase-activated receptor 1 activation induces epithelial apoptosis and increases intestinal permeability. Proc Natl Acad Sci USA 2003; 100: 11104–11109.
Babich M, King KL, Nissenson RA. Thrombin stimulates in-ositol phosphate production and intracellular free calcium by a pertussis toxin-insensitive mechanism in osteosarcoma cells. Endocrinology 1990; 126: 948–954.
Hung DT, Wong YH, Vu TK, Coughlin SR. The cloned platelet thrombin receptor couples to at least two distinct effectors to stimulate phosphoinositide hydrolysis and inhibit adenylyl cyclase. J Biol Chem 1992; 267: 20831–20834.
Chalmers CJ, Balmanno K, Hadfield K, Ley R, Cook SJ. Thrombin inhibits Bim (Bcl-2-interacting mediator of cell death) expression and prevents serum-withdrawal-induced apoptosis via protease-activated receptor 1. Biochem J 2003; 375: 99–109.
Du J, Brink M, Peng T, Mottironi B, Delafontaine P. Thrombin regulates insulin-like growth factor-1 receptor transcription in vascular smooth muscle: characterization of the signaling pathway. Circ Res 2001; 88: 1044–1052.
Hirota Y, Osuga Y, Yoshino O, et al. Possible roles of thrombin-induced activation of protease-activated receptor 1 in human luteinized granulosa cells. J Clin Endocrinol Metab 2003; 88: 3952–3957.
Lidington EA, Haskard DO, Mason JC. Induction of decay-accelerating factor by thrombin through a protease-activated receptor 1 and protein kinase C-dependent pathway protects vascular endothelial cells from complement-mediated injury. Blood 2000; 96: 2784–2792.
Mitsui H, Maruyama T, Kimura S, Takuwa Y. Thrombin activates two stress-activated protein kinases, c-Jun N-terminal kinase and p38, in HepG2 cells. Hepatology 1998; 27: 1362–1367.
Rahman A, True AL, Anwar KN, Ye RD, Voyno-Yasenetskaya TA, Malik AB. Galpha(q) and Gbetagamma regulate PAR-1 signaling of thrombin-induced NF-kappaB activation and ICAM-1 transcription in endothelial cells. Circ Res 2002; 91: 398–405.
Trejo J, Connolly AJ, Coughlin SR. The cloned thrombin receptor is necessary and sufficient for activation of mitogen-activated protein kinase and mitogenesis in mouse lung fibroblasts. Loss of responses in fibroblasts from receptor knockout mice. J Biol Chem 1996; 271: 21536–21541.
Wang H, Ubl JJ, Stricker R, Reiser G. Thrombin (PAR-1)-induced proliferation in astrocytes via MAPK involves multiple signaling pathways. Am J Physiol Cell Physiol 2002; 283: C1351–C1364.
Molloy CJ, Pawlowski JE, Taylor DS, Turner CE, Weber H, Peluso M. Thrombin receptor activation elicits rapid protein tyrosine phosphorylation and stimulation of the raf-1/MAP kinase pathway preceding delayed mitogenesis in cultured rat aortic smooth muscle cells: evidence for an obligate autocrine mechanism promoting cell proliferation induced by G-protein-coupled receptor agonist. J Clin Invest 1996; 97: 1173–1183.
Sabri A, Muske G, Zhang H, et al. Signaling properties and functions of two distinct cardiomyocyte protease-activated receptors. Circ Res 2000; 86: 1054–1061.
Carbajal JM, Gratrix ML, Yu CH, Schaeffer RC Jr. ROCK mediates thrombin's endothelial barrier dysfunction. Am J Physiol Cell Physiol 2000; 279: C195–C204.
Nguyen QD, Faivre S, Bruyneel E, et al. RhoA-and RhoD-dependent regulatory switch of Galpha subunit signaling by PAR-1 receptors in cellular invasion. FASEB J 2002; 16: 565–576.
Smirnova IV, Citron BA, Arnold PM, Festoff BW. Neuroprotective signal transduction in model motor neurons exposed to thrombin: G-protein modulation effects on neurite outgrowth, Ca(2+) mobilization, and apoptosis. J Neurobiol 2001; 48: 87–100.
Zimmermann KC, Green DR. How cells die: Apoptosis pathways. J Allergy Clin Immunol 2001; 108: S99–103.
Kawabata A. Gastrointestinal functions of proteinase-activated receptors. Life Sci 2003; 74: 247–254.
Xi G, Reiser G, Keep RF. The role of thrombin and thrombin receptors in ischemic, hemorrhagic and traumatic brain injury: Deleterious or protective? J Neurochem 2003; 84: 3–9.
Smirnova IV, Ma JY, Citron BA, et al. Neural thrombin and protease nexin I kinetics after murine peripheral nerve injury. J Neurochem 1996; 67: 2188–2199.
Akiyama H, Ikeda K, Kondo H, McGeer PL. Thrombin accumulation in brains of patients with Alzheimer's disease. Neurosci Lett 1992; 146: 152–154.
Striggow F, Riek-Burchardt M, Kiesel A, et al. Four different types of protease-activated receptors are widely expressed in the brain and are up-regulated in hippocampus by severe ischemia. Eur J Neurosci 2001; 14: 595–608.
Citron BA, Smirnova IV, Arnold PM, Festoff BW. Upregulation of neurotoxic serine proteases, prothrombin, and protease-activated receptor 1 early after spinal cord injury. J Neurotrauma 2000; 17: 1191–1203.
Beecher KL, Andersen TT, Fenton JW, Festoff BW. Thrombin receptor peptides induce shape change in neonatal murine astrocytes in culture. J Neurosci Res 1994; 37: 108–115.
Gurwitz D, Cunningham DD. Thrombin modulates and reverses neuroblastoma neurite outgrowth. Proc Natl Acad Sci USA 1988; 85: 3440–3444.
Zoubine MN, Ma JY, Smirnova IV, Citron BA, Festoff BW. A molecular mechanism for synapse elimination: novel inhibition of locally generated thrombin delays synapse loss in neonatal mouse muscle. Dev Biol 1996; 179: 447–457.
Vaughan PJ, Pike CJ, Cotman CW, Cunningham DD. Thrombin receptor activation protects neurons and astrocytes from cell death produced by environmental insults. J Neurosci 1995; 15: 5389–5401.
Lai JM, Hsieh CL, Chang ZF. Caspase activation during phorbol ester-induced apoptosis requires ROCK-dependent myosin-mediated contraction. J Cell Sci 2003; 116: 3491–3501.
Donovan FM, Cunningham DD. Signaling pathways involved in thrombin-induced cell protection. J Biol Chem 1998; 273: 12746–12752.
Turgeon VL, Milligan CE, Houenou LJ. Activation of the protease-activated thrombin receptor (PAR)-1 induces motoneuron degeneration in the developing avian embryo. J Neuropathol Exp Neurol 1999; 58: 499–504.
Masada T, Xi G, Hua Y, Keep RF. The effects of thrombin preconditioning on focal cerebral ischemia in rats. Brain Res 2000; 867: 173–179.
Xi G, Keep RF, Hua Y, Hoff JT. Thrombin preconditioning, heat shock proteins and thrombin-induced brain edema. Acta Neurochir Suppl 2000; 76: 511–515.
Striggow F, Riek M, Breder J, Henrich-Noack P, Reymann KG, Reiser G. The protease thrombin is an endogenous mediator of hippocampal neuroprotection against ischemia at low concentrations but causes degeneration at high concentrations. Proc Natl Acad Sci USA 2000; 97: 2264–2269.
Guo H, Liu D, Gelbard H, et al. Activated protein C prevents neuronal apoptosis via protease activated receptors 1 and 3. Neuron 2004; 41: 563–572.
Esmon CT. The protein C pathway. Chest 2003; 124: 26S–32S.
Taylor FB Jr, Chang A, Esmon CT, D'Angelo A, Vigano-D'Angelo S, Blick KE. Protein C prevents the coagulopathic and lethal effects of Escherichia coli infusion in the baboon. J Clin Invest 1987; 79: 918–925.
Bernard GR, Vincent JL, Laterre PF, et al. Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med 2001; 344: 699–709.
Kuliopulos A, Covic L, Seeley SK, Sheridan PJ, Helin J, Costello CE. Plasmin desensitization of the PAR1 thrombin receptor: kinetics, sites of truncation, and implications for thrombolytic therapy. Biochemistry 1999; 38: 4572–4585.
Cheng T, Liu D, Griffin JH, et al. Activated protein C blocks p53-mediated apoptosis in ischemic human brain endothelium and is neuroprotective. Nat Med 2003; 9: 338–342.
Riewald M, Petrovan RJ, Donner A, Mueller BM, Ruf W. Activation of endothelial cell protease activated receptor 1 by the protein C pathway. Science 2002; 296: 1880–1882.
Mosnier LO, Griffin JH. Inhibition of staurosporine-induced apoptosis of endothelial cells by activated protein C requires protease-activated receptor-1 and endothelial cell protein C receptor. Biochem J 2003; 373: 65–70.
McNamara CA, Sarembock IJ, Gimple LW, Fenton JW, Coughlin SR, Owens GK. Thrombin stimulates proliferation of cultured rat aortic smooth muscle cells by a proteolytically activated receptor. J Clin Invest 1993; 91: 94–98.
Shapiro PS, Evans JN, Davis RJ, Posada JA. The seven-transmembrane-spanning receptors for endothelin and thrombin cause proliferation of airway smooth muscle cells and activation of the extracellular regulated kinase and c-Jun NH2-terminal kinase groups of mitogen-activated protein kinases. J Biol Chem 1996; 271: 5750–5754.
Chambers RC, Leoni P, Blanc-Brude OP, Wembridge DE, Laurent GJ. Thrombin is a potent inducer of connective tissue growth factor production via proteolytic activation of protease-activated receptor-1. J Biol Chem 2000; 275: 35584–35591.
Sabri A, Short J, Guo J, Steinberg SF. Protease-activated receptor-1-mediated DNA synthesis in cardiac fibroblast is via epidermal growth factor receptor transactivation: distinct PAR-1 signaling pathways in cardiac fibroblasts and cardiomyocytes. Circ Res 2002; 91: 532–539.
Nierodzik ML, Kajumo F, Karpatkin S. Effect of thrombin treatment of tumor cells on adhesion of tumor cells to platelets in vitro and tumor metastasis in vivo. Cancer Res 1992; 52: 3267–3272.
Nierodzik ML, Chen K, Takeshita K, et al. Protease-activated receptor 1 (PAR-1) is required and rate-limiting for thrombin-enhanced experimental pulmonary metastasis. Blood 1998; 92: 3694–3700.
Gouin-Thibault I, Achkar A, Samama MM. The thrombophilic state in cancer patients. Acta Haematol 2001; 106: 33–42.
Wojtukiewicz MZ, Sierko E, Klement P, Rak J. The hemo-static system and angiogenesis in malignancy. Neoplasia 2001; 3: 371–384.
Lind SE, Caprini JA, Goldshteyn S, Dohnal JC, Vesely SK, Shevrin DH. Correlates of thrombin generation in patients with advanced prostate cancer. Thromb Haemost 2003; 89: 185–189.
Even-Ram S, Uziely B, Cohen P, et al. Thrombin receptor overexpression in malignant and physiological invasion processes. Nat Med 1998; 4: 909–914.
Gao C, Liu S, Hu HZ, et al. Serine proteases excite myenteric neurons through protease-activated receptors in guinea pig small intestine. Gastroenterology 2002; 123: 1554–1564.
Kawabata A, Nishikawa H, Saitoh H et al. A protective role of protease-activated receptor 1 in rat gastric mucosa. Gastroenterology 2004; 126: 208–219.
Wang J, Zheng H, Ou X, Fink LM, Hauer-Jensen M. Deficiency of microvascular thrombomodulin and up-regulation of protease-activated receptor-1 in irradiated rat intestine: possible link between endothelial dysfunction and chronic radiation fibrosis. Am J Pathol 2002; 160: 2063–2072.
Chin AC, Teoh DA, Scott KG, Meddings JB, MacNaughton WK, Buret AG. Strain-dependent induction of enterocyte apoptosis by Giardia lamblia disrupts epithelial barrier function in a caspase-3-dependent manner. Infect Immun 2002; 70: 3673–3680.
Scott KG, Meddings JB, Kirk DR, Lees-Miller SP, Buret AG. Intestinal infection with Giardia spp. reduces epithelial barrier function in a myosin light chain kinase-dependent fashion. Gastroenterology 2002; 123: 1179–1190.
Taggart CC, Greene CM, Smith SG, et al. Inactivation of human beta-defensins 2 and 3 by elastolytic cathepsins. J Immunol 2003; 171: 931–937.
Moncada D, Keller K, Chadee K. Entamoeba histolytica cysteine proteinases disrupt the polymeric structure of colonic mucin and alter its protective function. Infect Immun 2003; 71: 838–844.
Denecker G, Declercq W, Geuijen CA, et al. Yersinia ente-rocolitica YopP-induced apoptosis of macrophages involves the apoptotic signaling cascade upstream of bid. J Biol Chem 2001; 276: 19706–19714.
Forney JR, Yang S, Healey MC. Protease activity associated with excystation of Cryptosporidium parvum oocysts. J Parasitol 1996; 82: 889–892.
Rosenthal PJ. Proteases of protozoan parasites. Adv Parasitol 1999; 43: 105–159.
Williams AG, Coombs GH. Multiple protease activities in Giardia intestinalis trophozoites. Int J Parasitol 1995; 25: 771–778.
Jacobs T, Bruchhaus I, Dandekar T, Tannich E, Leippe M. Isolation and molecular characterization of a surface-bound proteinase of Entamoeba histolytica. Mol Microbiol 1998; 27: 269–276.
Huston CD, Houpt ER, Mann BJ, Hahn CS, Petri WA Jr. Caspase 3-dependent killing of host cells by the parasite Entamoeba histolytica. Cell Microbiol 2000; 2: 617–625.
Seydel KB, Li E, Zhang Z, Stanley SL Jr. Epithelial cell-initiated inflammation plays a crucial role in early tissue damage in amebic infection of human intestine. Gastroenterology 1998; 115: 1446–1453.
Buret AG, Chin AC, Scott KG. Infection of human and bovine epithelial cells with Cryptosporidium andersoni induces apoptosis and disrupts tight junctional ZO-1: Effects of epidermal growth factor. Int J Parasitol 2003; 33: 1363–1371.
McCole DF, Eckmann L, Laurent F, Kagnoff MF. Intestinal epithelial cell apoptosis following Cryptosporidium parvum infection. Infect Immun 2000; 68: 1710–1713.
Seydel KB, Stanley SL Jr. Entamoeba histolytica induces host cell death in amebic liver abscess by a non-Fas-dependent, non-tumor necrosis factor alpha-dependent pathway of apoptosis. Infect Immun 1998; 66: 2980–2983.
Lourbakos A, Chinni C, Thompson P, et al. Cleavage and activation of proteinase-activated receptor-2 on human neutrophils by gingipain-R from Porphyromonas gingivalis. FEBS Lett 1998; 435: 45–48.
Lourbakos A, Yuan YP, Jenkins AL, et al. Activation of protease-activated receptors by gingipains from Porphyromonas gingivalis leads to platelet aggregation: a new trait in microbial pathogenicity. Blood 2001; 97: 3790–3797.
Lourbakos A, Potempa J, Travis J et al. Arginine-specific protease from Porphyromonas gingivalis activates protease-activated receptors on human oral epithelial cells and induces interleukin-6 secretion. Infect Immun 2001; 69: 5121–5130.
Sun G, Stacey MA, Schmidt M, Mori L, Mattoli S. Interaction of mite allergens Der p3 and Der p9 with protease-activated receptor-2 expressed by lung epithelial cells. J Immunol 2001; 167: 1014–1021.
Asokananthan N, Graham PT, Stewart DJ, et al. House dust mite allergens induce proinflammatory cytokines from respiratory epithelial cells: The cysteine protease allergen, Der p 1, activates protease-activated receptor (PAR)-2 and inactivates PAR-1. J Immunol 2002; 169: 4572–4578.
Hong JH, Lee SI, Kim KE, et al. German cockroach extract activates protease-activated receptor 2 in human airway epithelial cells. J Allergy Clin Immunol 2004; 113: 315–319.
Page K, Strunk VS, Hershenson MB. Cockroach proteases increase IL-8 expression in human bronchial epithelial cells via activation of protease-activated receptor (PAR)-2 and extracellular-signal-regulated kinase. J Allergy Clin Immunol 2003; 112: 1112–1118.
McRedmond JP, Harriott P, Walker B, Fitzgerald DJ. Streptokinase-induced platelet activation involves anti-streptokinase antibodies and cleavage of protease-activated receptor-1. Blood 2000; 95: 1301–1308.
Fitzgerald DJ. Platelet activation in the pathogenesis of unstable angina: importance in determining the response to plasminogen activators. Am J Cardiol 1991; 68: 51B–57B.
Bansal A, Costa J, Habib I et al. Intestinal proteases modulate fluid secretion during Rotavirus infection. Gastroenterology 2004; 126: A–561.
Buresi MC, Buret AG, Hollenberg MD, MacNaughton WK. Activation of proteinase-activated receptor 1 stimulates epithelial chloride secretion through a unique MAP kinase-and cyclo-oxygenase-dependent pathway. FASEB J 2002; 16: 1515–1525.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Flynn, A.N., Buret, A.G. Proteinase-activated receptor 1 (PAR-1) and cell apoptosis. Apoptosis 9, 729–737 (2004). https://doi.org/10.1023/B:APPT.0000045784.49886.96
Issue Date:
DOI: https://doi.org/10.1023/B:APPT.0000045784.49886.96