Abstract
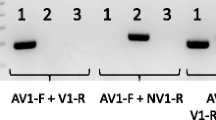
Rhodococcus equi is a facultative pathogen of foals. Infection causes an often fatal pulmonary pneumonia. The organism has also been isolated from pigs, cattle, humans and the environment. Equine virulence has a high positive correlation with the expression of a 17.4 kD polypeptide of unknown function, VapA, the product of the plasmid-encoded vapA gene. More recently an isogene of vapA, referred to as vapB and encoding an 18.2 kDa polypeptide, has been identified among pig and human isolates. The two genes share > 80% sequence identity, yet their host strains apparently exhibit different pathogenicity profiles (for example by reference to virulence in mouse model system and host specificity). In this study, a polymerase chain reaction (PCR) technique was developed that permits the selective amplification of vapA and vapB. Using this technique the distribution of the two genes among 35 randomly selected isolates of Rhodococcus equi from various animal and environmental sources was determined. Using this technique the genotype of each isolate could be unambiguously assigned as vapA +, vapB + or vap − (i.e., scoring negative for both vapA and vapB). No isolate scored positive for both vapA and vapB. 100% of equine isolates scored vapA +, confirming the status of vapA as a reliable marker of equine virulence. All three genotypes were found among human isolates; porcine isolates scored either vapB + or vap − and no vapA + isolates were present in this sample. Rigorous statistical analysis using the Fisher Exact test confirmed that the high frequency of vapA + among equine isolates is significant; however the sample size was too small to draw statistically significant conclusions regarding the distribution of genotypes among within other animal groups.
Similar content being viewed by others
References
Arriaga J.M., Cohen N.D., Derr J.N., Chaffin K and Martens R.J. 2002. Detection of Rhodococcus equi by polymerase reaction using species-specific non-proprietary primers. J. Vet. Diagn. In-vest. 14: 347–353.
Byrne B.A., Prescott J.F., Palmer G.H., Takai S, Nicholson V.M., Alperin D.C. and Hines S.A. 2001. Virulence plasmid of Rhodo-coccus equi contains inducible gene family encoding several proteins. Infect. Immun. 69: 650–656.
Drancourt M., Bonnet E., Gallais H., Peloux Y. and Raoult D. 1992. Rhodococcus equi infection in patients with AIDS. J. Infect. 24: 123–131.
Flynn O., Quigley F., Costello E., O'Grady D., Gogarty A., McGuirk J. and Takai S. 2001. Virulence-associated protein characterisation of Rhodococcus equi isolated from bovine lymph nodes. Vet. Microbiol. 78: 221–228.
Golub B., Falk G. and Spink W.W., 1967. Lung abscess due to Corynebacterium equi, report of first human infection. Ann. Int. Med. 66: 1174–1177.
Goodfellow M., Beckham AR. and Barton M.D. 1982. Numerical classification of Rhodococcus equi and related actinomycetes. J. Appl. Bacteriol. 53: 199–207.
Haites R.E., Muscatello G., Begg A.P. and Browning G.F. 1997. Prevalence of the virulence-associated gene of Rhodococcus equi in isolates from infected foals. J. Clin. Microbiol. 35: 1642–1644.
Johnson J.A., Prescott J.F. and Markham K.J. 1983a. The pathology of experimental Corynebacterium equi infection in foals following intrabronchial challenge. Vet. Path. 20: 440–449.
Johnson J.A., Prescott J.F. and Markham K.J. 1983b. The pathology of experimental Corynebacterium equi infection in foals following intragastric challenge. Vet. Path. 20: 450–459.
Kedlaya I., Ing M.B. and Wong SS. 2001. Rhodococcus equi infections in immunocompetent hosts: case report and review. Clin. Infect. Dis 32: 39–46.
Ladrón N., Fernández M., Agüero J., Zörn B.G., Vásquez-Boland J.A. and Navas J. 2003. Rapid identification of Rhodococcus equi by a PCR assay targeting the choE gene. J. Clin. Microbiol. 41: 3241–3245.
Madarame H., Yaegashi R., Fukunga N., Matsukuma M., Mutoh K., Morisawa N., Sasaki Y., Tsubaki S., Hasagawa Y. and Takai S. 1998. Pathogenicity of Rhodococcus equi strains possessing virulence-associated 15-to 17kDa and 20kDa antigens: experimental and natural case in pigs. J. Comp. Path 119: 397–405.
Magnusson H. 1923. Spezifische infektioese pneumonie biem Fohelen. Ein neuer Eitererreger beim Pferd. Arch. Wiss. Prakt. Tierheilkunde 50: 22–28.
Makrai L., Takai S., Tamura A., Tsukamoto A., Sekimoto R., Sasaki Y., Kakuda T., Tsubaki S., Varga J., Fodor L., Solymosi N. and Major A. 2002. Characterization of virulence plasmid types in Rhodococcus equi isolates from foals, pigs, humans and soil in Hungary. Vet. Microbiol. 88: 377–384.
Martens R.J., Fiske R.A. and Renshaw H.W. 1982. Experimental subacute foal pneumonia inducible by aerosol administration of Corynebacterium equi. Equine Vet. J. 14: 111–116.
McNeil M.M. and Brown J.M. 1994. The medically important aerobic actinomycetes: epidemiology and microbiology. Clin. Microbiol. Rev. 7: 357–417.
Morton A.C., Begg A.P., Anderson G.A., Takai S., Lämmler C. and Browning G.F. 2001. Epidemiology of Rhodococcus equi strains on thoroughbred horse farms. Appl. Environ. Microbiol. 67: 2167–2175.
Mosser D.M. and Hondulas M.K. 1996. Rhodococcus equi:an emerging opportunistic pathogen. Trends in Microbiology. 4: 29–33.
Prescott J.F. 1991. Rhodococcus equi: an animal and human pathogen. Clin. Microbiol. Rev. 4: 20–34.
Sekizaki T., Takai S., Hguiya Y., Ikeda Y., Ito H. and Tsubaki S. 1995. Sequence of Rhodococcus equi gene encoding for the virulence-associated 15–17 kDa antigens. Gene 155: 135–136.
Scott M.A., Graham B.S., Verrall R., Dixon R., Schaffner W. and Tham K.T. 1995. Rhodococcus equi an increasingly recognized opportunistic pathogen. Clin. Microbiol. Infect. Dis. 103: 649–655.
Sellon D.C., Besser T.E., Vivrette S.L. and McConnico R.S. 2001. Comparison of nucleic acid amplification, serology and micro-biologic culture for diagnosis of Rhodococcus equi in foals. J. Clin. Microbiol. 39: 1289–1293.
Takai S., Kawazu S. and Tsubaki S. 1987. Humoral immune response of foals to experimental infection with Rhodococcus equi. Vet. Microbiol. 14: 321–327.
Takai S., Sekizaki T., Osawa T., Sugawara T., Wanatabe Y. and Tsubaki S. 1991a. Association between a large plasmid and 15-to 17-kilodalton antigens in virulent Rhodococcus equi. Infect. Immun. 59: 4056–4060.
Takai S., Imai Y., Fukunaga N., Uchida Y., Kamisawa K., Sasaki Y., Tsubaku S. and Sekizaki T. 1991b. Identification of 15-to 17-kilodalton antigens associated with virulent Rhodococcus equi. J. Clin. Microbiol. 29: 439–443.
Takai S., Lie M., Wanatabe S., Tsubaki S. and Sekizaki T. 1992. Virulence-associated 15-to 17 kDa antigens in Rhodococcus equi: temperature-dependent expression and location of antigens. Infect. Immun. 60: 2995–2997.
Takai S., Sasaki Y., Ikeda T., Uchida T., Tsubaki S. and Sekizaki T. 1994. Virulence of Rhodococcus equi from patients with and without AIDS. J. Clin. Microbiol. 32: 457–60.
Takai S., Imai Y., Fukunaga N., Uchida Y., Kamisawa K., Sasaki Y., Tsubaki S. and Sekizaki T. 1995. Identification of virulence-associated antigens and plasmids in Rhodococcus equi from patients with AIDS. J. Infect. Dis. 172: 1306–1311.
Takai S., Fukanaga N., Ochiai S., Imasi Y., Sasaki Y., Tsubaki S. and Sekizaki T. 1996a. Identification of intermediately virulent Rhodococcus equi isolates from pigs. J. Clin. Microbiol. 34: 1034–1037.
Takai S., Fukunaga N., Kamisawa K., Imai Y., Sasaki Y. and Tsubaki S. 1996b. Expression of virulence associated antigens of Rhodococcus equi is regulated by temperature and pH. Microbial. Immunol. 40: 591–594.
Takai S., Vigo C., Ikushima H., Higuchi T., Hagiwara S-T., Hashikura S., Sasaki Y., Tsubaki S., Anazi T. and Kamada M. 1998. Detection of virulent Rhodococcus equi in tracheal aspirate samples by polymerase chain reaction for rapid diagnosis of R. equi pneumonia in foals. Vet. Microbiol. 61: 51–58.
Takai S., Hines S.A., Sekizaki T., Nicholson V., Alperin D.A., Osaki M., Takamatsu D., Nakamura M., Suzuki K., Ogino N., Kakuda T., Dan H. and Prescott J.F. 2000a. DNA sequence and comparison of virulence plasmids from Rhodococcus equi ATCC 33701 and 103. Infect. Immun. 68: 6840–6847.
Takai S., Anzai T., Fujita Y., Akita O., Shoda M., Tsubaki S. and Wada R. 2000b. Pathogenicity of Rhodococcus equi expressing a virulence-associated 20 kD protein (VapB) in foals. Vet. Microbiol. 76: 71–80.
Tkachuk-Saad O. and Prescott J.F. 1991. Rhodococcus equi plasmids: Isolation and partial characterisation. J. Clin. Microbiol. 29: 2696–2700.
Vivrette S.L., Sellon D.C. and Gibbons D.S. 2000. Clinical application of a polymerase chain reaction assay in the diagnosis of pneumonia caused by Rhodococcus equi in a horse. JAVMA217: 1348–1350.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Oldfield, C., Bonella, H., Renwick, L. et al. Rapid determination of vapA/vapB genotype in Rhodococcus equi using a differential polymerase chain reaction method. Antonie Van Leeuwenhoek 85, 317–326 (2004). https://doi.org/10.1023/B:ANTO.0000020383.66622.4d
Issue Date:
DOI: https://doi.org/10.1023/B:ANTO.0000020383.66622.4d