Abstract
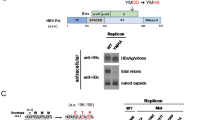
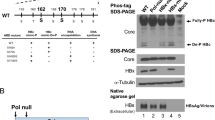
Although the structure–function of Hepatitis B virus (HBV) core protein has been investigated by numerous HBV core mutants, functions of many regions in the core protein are still remained to be identified. In this report, it was found that point mutations in the 113th and 117th negative-charged amino acids in the 5th helix region of the HBV core strongly affect pregenomic RNA (pgRNA) encapsidation. These mutations were introduced by site-directed mutagenesis. The following results were obtained from analyses of the mutants. First, endogenous polymerase activity (EPA) was assayed and activity was not detected only in the two mutants, E113K and E117K. Second, the pgRNA encapsidation level of each mutant related to a change in charge of two amino acid sites was evaluated. Mutations in the 113th and 117th amino acids into uncharged amino acids reduced pgRNA encapsidation levels. Moreover, changes of the two amino acids into positive-charged amino acids almost completely reduced pgRNA encapsidation levels. To test whether the mutant core proteins assembled into normal capsid particles, the assembly of the mutant core proteins was seen. However, none of the changes in the 113th and 117th amino acids affected capsid formation. From this data, it can be inferred that the above two amino acids in the 5th alpha-helix in the HBV core protein are important for pgRNA encapsidation.
Similar content being viewed by others
References
Summers J. and Mason W.S., Cell 29, 403-415, 1982.
Ganem D. and Varmus H.E., Annu Rev Biochem 56, 651-693, 1987.
Nassal M. and Schaller H., Trends Microbiol 1, 221-228, 1993.
Nassal M., Curr Topics Microbiol Immunol 214, 297-337, 1996.
Hirsch R.C., Lavine J.E., Chang L.J., Varmus H.E., and Ganem D., Nature 344, 552-555, 1990.
Junker-Niepmann M., Bartenschlager R., and Schaller H., EMBO J 9(10), 3389-3396, 1990.
Wang G.H. and Seeger C., Cell 71, 663-670, 1992.
Seifer M. and Standring D.N., J Virol 67, 4513-4520, 1993.
Tavis J.E. and Ganem D., Proc Natl Acad Sci USA 90, 4107-4111, 1993.
Nassal M., J Virol 66, 4107-4116, 1992.
Schlicht H.J., Bartenschlager R., and Schaller H., J Virol 63, 2995-3000, 1989.
Yu M. and Summers J., J Virol 65, 2511-2517, 1991.
Tiollais P., Charnay P., and Vyas G.N., Science 213, 406-411, 1981.
Gallina A., Bonelli F., Zentilin L., Rindi G., Muttini M., and Milanesi G., J Virol 63, 4645-4652, 1989.
Birnbaum F. and Nassal M., J Virol 64, 3319-3330, 1990.
Zheng J., Schodel F., and Peterson D.L., J Biol Chem 267, 9422-9429, 1992.
Hatton T., Zhou S., and Standring D.N., J Virol 66, 5232-5241, 1992.
Scaglioni P.P., Melegari M., and Wands J.R., Virology 205, 112-120, 1994.
Koschel M., Thomssen R., and Bruss V., J Virol 73, 2153-2160, 1999.
Koschel M., Oed D., Gerelsaikhan T., Thomssen R., and Bruss V., J Virol 74, 1-7, 2000.
Shih C., Li L.S., Roychoudhuary S., and Ho M.H., Proc Natl Acad Sci USA 86, 6323-6327, 1989.
Bruss V. and Ganem D., Proc Natl Acad Sci USA 88, 1059-1063, 1991.
Yuan T.T., Faruqi A., Shih J.W.K., and Shih C., Virology 211, 144-156, 1995.
Suk F.M., Lin M.H., Newman M., Pan S., Chen S.H., Liu J.D., and Shih C., J Virol 76(23), 12069-12077, 2002.
Yuan T.T., Sahu G.K., Whitehead W., Greenberg R., and Shih C., J Virol 73, 5731-5740, 1999.
Beames B. and Lanford R.E., J Virol 69, 6833-6838, 1995.
Yuan T.T., Lin M.H., Qiu S.M., and Shih C., J Virol 72, 2168-2176, 1998.
Böttcher B., Wynne S.A., and Crowther R.A., Nature 386, 88-91, 1997.
Wynne S.A., Crowther R.A., and Leslie A.G.W., Mol Cell 3, 771-780, 1999.
von Weizäscker F., Köck J., Wieland S., Beck J., Nassal M., and Blum H.E., Hepatology 35, 209-216, 2002.
Bartenschlager R., Junker-Niepmann M., and Schaller H., J Virol 64, 5324-5332, 1990.
Gazina E.V., Fielding J.E., Lin B., and Anderson D.A., J Virol 74, 4721-4728, 2000.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, S.M., Park, S.G., Park, E. et al. The 113th and 117th Charged Amino Acids in the 5th Alpha-Helix of the HBV Core Protein are Necessary for pgRNA Encapsidation. Virus Genes 27, 227–235 (2003). https://doi.org/10.1023/A:1026339731001
Issue Date:
DOI: https://doi.org/10.1023/A:1026339731001