Abstract
Purpose. To use an inverse gas chromatographic (IGC) method to determine the glass transition temperature (Tg) of some amorphous pharmaceuticals and to extend this technique for the in situ study of the plasticizing effect of water on these materials.
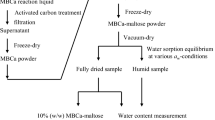
Methods. Amorphous sucrose and colyophilized sucrose-PVP mixtures were the model compounds. Both IGC and differential scanning calorimetry (DSC) were used to determine their Tg. By controlling the water vapor pressure in the IGC sample column, it was possible to determine the Tg of plasticized amorphous phases. Under identical temperatures and vapor pressures, the water uptake was independently quantified in an automated water sorption apparatus.
Results. The Tg of the dry phases, determined by IGC and by DSC, were in very good agreement. With an increase in the environmental relative humidity (RH), there was a progressive decrease in Tg as a result of the plasticizing effect of water. Because the water uptake was independently quantified, it was possible to use the Gordon-Taylor equation to predict the Tg values of the plasticized materials. The predicted values were in very good agreement with those determined experimentally using IGC. A unique advantage of this technique is that it provides complete control over the sample environment and is thus ideally suited for the characterization of highly reactive amorphous phases.
Conclusions. An IGC method was used (a) to determine the glass transition temperature of amorphous pharmaceuticals and (b) to quantify the plasticizing effect of water on multicomponent systems.
Similar content being viewed by others
References
H. P. Schreiber and D. R. Lloyd. Overview of inverse gas chromatography. In D. R. Lloyd, T. C. Ward, and H. P. Schreiber (eds.), Inverse Gas Chromatography: Characterization of Polymers and Other Materials, American Chemical Society, Washington, DC, 1989, pp.1-10.
D. Cline and R. Dalby. Predicting the quality of powders for inhalation from surface energy and area. Pharm. Res. 19:1274-1277 (2002).
J. C. Feeley, P. York, B. S. Sumby, and H. Dicks. Determination of surface properties and flow characteristics of salbutamol sulfate, before and after micronization. Int. J. Pharm. 172:89-96 (1998).
M. D. Ticehurst. Characterisation of the surface energetics of pharmaceutical powders by inverse gas chromatography, Ph.D. Thesis, University of Bradford, UK, 1995.
G. Buckton. Characterization of small changes in the physical properties of powders of significance for dry powder inhaler formulations. Adv. Drug Delivery Rev. 26:17-27 (1997).
H. E. Newell, G. Buckton, D. A. Butler, F. Thielmann, and D. R. Williams. The use of inverse phase gas chromatography to measure the surface energy of crystalline, amorphous, and recently milled lactose. Pharm. Res. 18:662-666 (2001).
H. E. Newell, G. Buckton, D. A. Butler, F. Thielmann, and D. R. Williams. The use of inverse phase gas chromatography to study the change of surface energy of amorphous lactose as a function of relative humidity and the processes of collapse and crystallization. Int. J. Pharm. 217:45-56 (2001).
P. York, M. D. Ticehurst, J. C. Osborn, R. J. Roberts, and R. C. Rowe. Characterization of the surface energetics of milled dl-propranolol hydrochloride using inverse gas chromatography and molecular modeling. Int. J. Pharm. 174:179-186 (1998).
L. Yu. Amorphous pharmaceutical solids: Preparation, characterization and stabilization. Adv. Drug Delivery Rev. 48:27-42 (2001).
B. C. Hancock and G. Zografi. Characteristics and significance of the amorphous state in pharmaceutical systems. J. Pharm. Sci. 86:1-12 (1997).
D. Q. M. Craig, P. G. Royall, V. L. Kett, and M. L. Hopton. The relevance of the amorphous state to pharmaceutical dosage forms: Glassy drugs and freeze dried systems. Int. J. Pharm. 179:179-207 (1999).
J. Kerc and S. Srcic. Thermal analysis of glassy pharmaceuticals. Thermochim. Acta 248:81-95 (1995).
B. C. Hancock and G. Zografi. Relationship between the glass transition temperature and the water content of amorphous pharmaceutical solids. Pharm. Res. 11:471-477 (1994).
M. Gordon and J. S. Taylor. Ideal copolymers and the second-order transitions of synthetic rubbers. I. Non-crystalline copolymers. J. Appl. Chem. 2:493-500 (1952).
Y. Roos. Melting and glass transitions of low molecular weight carbohydrates. Carbohydr. Res. 238:39-48 (1993).
L. S. Taylor and G. Zografi. Sugar-polymer hydrogen bond interactions in lyophilized amorphous mixtures. J. Pharm. Sci. 87:1615-1621 (1998).
V. L. Hill, D. Q. M. Craig, and L. C. Feely. Characterization of spray-dried lactose using modulated differential scanning calorimetry. Int. J. Pharm. 161:95-107 (1998).
J. E. Guillet, M. Romansky, G. J. Price, and R. Van der Mark. Studies of polymer structure and interactions by automated inverse gas chromatography. In D. R. Lloyd, T. C. Ward, and H. P. Schreiber (eds.), Inverse Gas Chromatography: Characterization of Polymers and Other Materials, American Chemical Society, Washington, DC, 1989, pp.20-32.
F. Thielmann and D. Williams. Determination of the glass transition temperature of maltose and its dependence on relative humidity by inverse gas chromatography. Dtsch. Lebensm.-Rundsch. 96:255-257 (2000).
J. R. Conder and C. L. Young. Physicochemical Measurement by Gas Chromatography, John Wiley & Sons, Chichester, 1979.
A. V. Kiselev and Y. I. Yashin. Gas Adsorption Chromatography, Plenum Press, London, 1969.
A. E. Bolvari, T. C. Ward, P. A. Koning, and D. P. Sheehy. Experimental techniques for inverse gas chromatography. In D. R. Lloyd, T. C. Ward, and H. P. Schreiber (eds.), Inverse Gas Chromatography: Characterization of Polymers and Other Materials, American Chemical Society, Washington, DC, 1989, pp.12-19.
J. F. Parcher. Fundamental relationships in gas chromatography. Chromatographia 47:570-574 (1998).
J. M. Braun and J. E. Guillet. Studies of polystyrene in the region of the glass transition temperature by inverse gas chromatography. Macromolecules 8:882-888 (1975).
S. L. Shamblin, L. S. Taylor, and G. Zografi. Mixing behavior of colyophilized binary systems. J. Pharm. Sci. 87:694-701 (1998).
C. T. Moynihan, A. J. Easteal, and J. Wilder. and J. Tucker. Dependence of the glass transition temperature on heating and cooling rate. J. Phys. Chem. 78:2673-2677 (1974).
R. Surana and R. Suryanarayanan. Quantitation of crystallinity in substantially amorphous pharmaceuticals and study of crystallization kinetics by X-ray powder diffractometry. Powder Diffr. 15:2-6 (2000).
O. Smidsroed and J. E. Guillet. Study of polymer–solute interactions by gas chromatography. Macromolecules 2:272-277 (1969).
R. R. Edwards, Y. Tao, S. Xu, P. S. Wells, K. S. Yun, and J. F. Parcher. Chromatographic investigation of the effect of dissolved carbon dioxide on the glass transition temperature of a polymer and the solubility of a third component (additive). J. Polym. Sci. Part B: Polym. Phys. 36:2537-2549 (1998).
J. Fan, E. I. Cooper, and C. A. Angell. Glasses with strong calorimetric β-glass transitions and the relation to the protein glass transition problem. J. Phys. Chem. 98:9345-9349 (1994).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Surana, R., Randall, L., Pyne, A. et al. Determination of Glass Transition Temperature and in Situ Study of the Plasticizing Effect of Water by Inverse Gas Chromatography. Pharm Res 20, 1647–1654 (2003). https://doi.org/10.1023/A:1026199604374
Issue Date:
DOI: https://doi.org/10.1023/A:1026199604374