Abstract
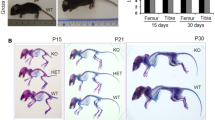
Biglycan is a Class I Small Leucine Rich Proteoglycans (SLRP) that is localized on human chromosome Xq28-ter. The conserved nature of its intron-exon structure and protein coding sequence compared to decorin (another Class I SLRP) indicates the two genes may have arisen from gene duplication. Biglycan contains two chondroitin sulfate glycosaminoglycan (GAG) chains attached near its NH2 terminus making it different from decorin that has only one GAG chain. To determine the functions of biglycan in vivo, transgenic mice were developed that were deficient in the production of the protein (knockout). These mice acquire diminished bone mass progressively with age. Double tetracycline-calcein labeling revealed that the biglycan deficient mice are defective in their capacity to form bone. Based on this observation, we tested the hypothesis that the osteoporosis-like phenotype is due to defects in cells critical to the process of bone formation. Our data shows that biglycan deficient mice have diminished capacity to produce marrow stromal cells, the bone cell precursors, and that this deficiency increases with age. The cells also have reduced response to tranforming growth factor-β (TGF-β), reduced collagen synthesis and relatively more apoptosis than cells from normal littermates. In addition, calvaria cells isolated from biglycan deficient mice have reduced expression of late differentiation markers such as bone sialoprotein and osteocalcin and diminished ability to accumulate calcium judged by alizerin red staining. We propose that any one of these defects in osteogenic cells alone, or in combination, could contribute to the osteoporosis observed in the biglycan knockout mice. Other data suggests there is a functional relationship between biglycan and bone morphogenic protein-2/4 (BMP 2/4) action in controlling skeletal cell differentiation. In order to test the hypothesis that functional compensation can occur between SLRPs, we created mice deficient in biglycan and decorin. Decorin deficient mice have normal bone mass while the double biglycan/decorin knockout mice have more severe osteopenia than the single biglycan indicating redundancy in SLRP function in bone tissue. To further determine whether compensation could occur between different classes of SLRPs, mice were generated that are deficient in both biglycan (class I) and fibromodulin, a class II SLRP highly expressed in mineralizing tissue. These doubly deficient mice had an impaired gait, ectopic calcification of tendons and premature osteoarthritis. Transmission electron microscopy analysis showed that like the decorin and biglycan knockouts, they have severely disturbed collagen fibril structures. Biomechanical analysis of the affected tendons showed they were weaker compared to control animals leading to the conclusion that instability of the joints could be the primary cause of all the skeletal defects observed in the fibromodulin/biglycan knockout mice. These studies present important new animal models for musculoskeletal diseases and provide the opportunity to characterize the network of signals that control tissue integrity and function through SLRP activity. Published in 2003.
Similar content being viewed by others
References
Fisher LW, Termine JD, Dejter SW Jr, Whitson SW, Yanagishita M, Kimura JH, Hascall VC, Kleinman HK, Hassell JR, Nilsson B, Proteoglycans of developing bone, J Biol Chem 258, 6588–94 (1983).
Fisher LW, Termine JD, Young MF, Deduced protein sequence of bone small proteoglycan I (biglycan) shows homology with proteoglycan II (decorin) and several nonconnective tissue proteins in a variety of species, J Biol Chem 264, 4571–6 (1989).
Iozzo R, The biology of the small leucine-rich proteoglycans, J Biol Chem 274, 18843–6 (1999).
Hocking AM, Shinomura T, McQuillan DJ, Leucine-rich repeat glycoproteins of the extracellular matrix, Matrix Biol 17, 1–19 (1998).
Ameye L, Young MF, Mice deficient in small leucine-rich proteoglycans: novel in vivo models for osteoporosis, osteoarthritis, Ehlers-Danlos syndrome, muscular dystrophy, and corneal diseases, Glycobiology 12, 107R–116R (2002).
Kresse H, Schonherr E, Proteoglycans of the extracellular matrix and growth control, J Cell Physiol 189, 266–74 (2001).
Scott IC, Imamura Y, Pappano WN, Troedel JM, Recklies AD, Roughley PJ, Greenspan DS, Bone morphogenetic protein-1 processes probiglycan, J Biol Chem 275, 30504–11 (2000).
Bianco P, Riminucci M, Fisher L, Dermatan Sulphate Proteoglycans (Portland Press, London, 1993), pp. 193–205.
Fisher L, Extracellular Matrix, Anchor, and Adhesion Proteins, (Oxford University Press Inc., New York, 1999), pp. 365–8.
McBride OW, Fisher LW, Young MF, Localization of PGI (biglycan, BGN) and PGII (decorin, DCN, PG-40) genes on human chromosomes Xq13-qter and 12q, respectively, Genomics 6, 219–25 (1990).
Traupe H, van den Ouweland AM, van Oost BA, Vogel W, Vetter U, Warren ST, Rocchi M, Darlison MG, Ropers HH, Fine mapping of the human biglycan (BGN) gene within the Xq28 region employing a hybrid cell panel, Genomics 13, 481–3 (1992).
Fisher LW, Heegaard AM, Vetter U, Vogel W, Just W, Termine JD, Young MF, Human biglycan gene. Putative promoter, intronexon junctions, and chromosomal localization, J Biol Chem 266, 14371–7 (1991).
Vetter U, Vogel W, Just W, Young MF, Fisher LW, Human decorin gene: Intron-exon junctions and chromosomal localization, Genomics 15, 161–8 (1993).
Geerkens C, Vetter U, Just W, Fedarko NS, Fisher LW, Young MF, Termine JD, Robey PG, Wohrle D, Vogel W, The X-chromosomal human biglycan gene BGN is subject to X inactivation but is transcribed like an X-Y homologous gene, Hum Genet 96, 44–52 (1995).
Heegaard AM, Gehron Robey P, Vogel W, Just W, Widom RL, Scholler J, Fisher LW, Young MF, Functional characterization of the human biglycan 5_-flanking DNA and binding of the transcription factor c-Krox, J Bone Miner Res 12, 2050–60 (1997).
Ungefroren H, Krull NB, Transcriptional regulation of the human biglycan gene, J Biol Chem 271, 15787–95 (1996).
Ellison JW, Wardak Z, Young MF, Gehron Robey P, Laig-Webster M, Chiong W, PHOG, a candidate gene for involvement in the short stature of Turner syndrome, Hum Mol Genet 6, 1341–7 (1997).
Bianco P, Fisher L, Young M, Termine J, Gehron Robey P, Expression and localization of the two small proteoglycans biglycan and decorin in developing human skeletal and non-skeletal tissues, J Histochem Cytochem 38, 1549–63 (1990).
Xu T, Bianco P, Fisher L, Longenecker G, Smith E, Goldstein S, Bonadio J, Boskey A, Heegaard A, Sommer B, Satomura K, Dominguez P, Zhao C, Kulkarni A, Gehron-Robey P, Young M, Targeted disruption of the biglycan gene leads to an osteoporosislike phenotype in mice, Nature Genet 20, 78–82 (1998).
Corsi A, Xu T, Chen XD, Boyde A, Liang J, Mankani M, Sommer B, Iozzo RV, Eichstetter I, Robey PG, Bianco P, Young MF, Phenotypic effects of biglycan deficiency are linked to collagen fibril abnormalities, are synergized by decorin deficiency, and mimic Ehlers-Danlos-like changes in bone and other connective tissues, J Bone Miner Res 17, 1180–9 (2002).
Chen XD, Shi S, Xu T, Robey PG, Young MF, Age-related osteoporosis in biglycan-deficient mice is related to defects in bone marrow stromal cells, J Bone Miner Res 17, 331–40 (2002).
Hildebrand A, Romaris M, Rasmussen L, Heinegard D, Twardzik D, Borders W, Ruoslathi E, Interaction of the small interstitial proteoglycans, decorin and fibromodulin with transforming growth factor b, Biochem J 302, 527–34 (1994).
Chen X-D, Xu T, Young MF, Biglycan is essential for BMP-4 stimulated osteoblast differentiation, J Bone Miner Res 17, S141 (2002).
Goldberg M, Rapoport O, Septier D, Palmier KRH, Embery G, Young M, Ameye L, Proteoglycans in predentin: The last 15 micrometers before mineralization, Conn Tiss Res 44(Suppl. 1), 184–88 (2003).
Bowe MA, Mendis DB, Fallon JR, The small leucine-rich repeat proteoglycan biglycan binds to alpha-dystroglycan and is upregulated in dystrophic muscle, J Cell Biol 148, 801–10 (2000).
Danielson K, Baribault H, Holmes D, Graham H, Kadler K, Iozzo R, Targeted disruption of decorin leads to abnormal collagen fibril morphology and skin fragility, J Cell Biol 136, 729–49 (1997).
Ameye L, Young MF, Chen XD, Wiley Encyclopedia of Molecular Medecine (Wiley & Sons, New York City, 2002), pp. 1008–10.
Bi Y, Chen X-D, Xu T, Iozzo R, Young M, Biglycan and Decorin have overlapping roles in the commitment, proliferation, and survival of osteoblasts and their precursors, J Bone Miner Res 17, S141 (2002).
Kresse H, Rosthoj S, Quentin E, Hollmann J, Glossl J, Okada S, Tonnesen T, Glycosaminoglycan-free small proteoglycan core protein is secreted by fibroblasts from a patient with a syndrome resembling progeroid, Am J Hum Genet 41, 436–53 (1987).
Ameye L, Aria D, Jepsen K, Oldberg A, Xu T, Young MF, Abnormal collagen fibrils in tendons of biglycan/fibromodulin-deficient mice lead to gait impairment, ectopic ossification, and osteoarthritis, Faseb J 16, 673–80 (2002).
Ameye L, Oliveira M, Offord E, Young MF, Oateoarthritis in the biglycan fibromodulin double defficient mouse is associated with increased level of cartilage oligomeric matrix protein and decreased levels of decorin and type II collegen in the articular cartilage, Osteoarthritis Cartilage 10(Suppl.), S6 (2002).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Young, M.F., Bi, Y., Ameye, L. et al. Biglycan knockout mice: New models for musculoskeletal diseases. Glycoconj J 19, 257–262 (2002). https://doi.org/10.1023/A:1025336114352
Issue Date:
DOI: https://doi.org/10.1023/A:1025336114352