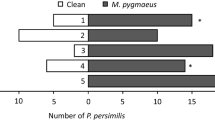
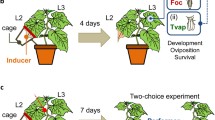
Abstract
Phytoseiid mites use herbivore-induced plant volatiles in long-range prey-habitat location and are arrested by these volatiles in a prey patch. The responses of predatory mites to these volatiles are considered to be an important factor in the local extermination of prey populations by phytoseiids such as Phytoseiulus persimilis. Prey-induced plant volatiles are highly detectable and can be reliable indicators of prey presence and prey identity. The composition of herbivore-induced plant volatiles depends on plant species and plant cultivar. Moreover, the composition may also vary with the herbivore species that infests a plant. The responses of phytoseiids to prey-induced plant volatiles from a specific plant-herbivore combination are highly variable. Causal factors include starvation, specific hunger, experience, pathogen infestation and the presence of competitors. Investigating variation in the phytoseiid's behavioural response in relation to these factors is important for understanding how and why behavioural strategies maximize phytoseiid fitness.
Similar content being viewed by others
REFERENCES
Bruin, J., Dicke, M. and Sabelis, M., 1992. Plants are better protected against spider-mites after exposure to volatiles from infested conspecifics. Experientia 48: 525–529.
Carmichael, L.M., Moore, J. and Bjostad, L.B. 1993. Parasitism and decreased response to sex pheromones in male Periplaneta americana (Dictyoptera: Blattidae). J. Insect Behav. 6: 25–32.
De Bruyne, M., Dicke, M. and Tjallingii, W.F., 1991. Receptor cell responses in the anterior tarsi of Phytoseiulus persimilis to volatile kairomone components. Exp. Appl. Acarol. 13: 53–58.
DeMoraes, G.J. and McMurtry, J.A. 1987. Physiological effect of the host plant on the suitability of Tetranychus urticae as prey for Phytoseiulus persimilis (Acari: Tetranychidae, Phytoseiidae). Entomophaga 32: 35–38.
Dicke, M., 1988. Prey preference of the phytoseiid mite Typhlodromus pyri: 1. Response to volatile kairomones. Exp. Appl. Acarol. 4: 1–13.
Dicke, M. 1998. Evolution of induced indirect defence of plants. In The Ecology and Evolution of Inducible defenses, C.D. Harvell and R. Tollrian (eds). Princeton University Press, in press.
Dicke, M. and Groeneveld, A. 1986. Hierarchical structure in kairomone preference of the predatory mite Amblyseius potentillae: dietary component indispensable for diapause induction affects prey location behaviour. Ecol. Entomol. 11: 131–138.
Dicke, M. and Sabelis M.W. 1988a. Infochemical terminology: based on cost-benefit analysis rather than origin of compounds? Funct. Ecol. 2: 131–139.
Dicke, M. and Sabelis, M.W. 1988b. How plants obtain predatory mites as bodyguards. Neth. J. Zool. 38: 148–165.
Dicke, M. and Vet, L.E.M. 1998. Plant-carnivore interactions: evolutionary and ecological consequences for plant, herbivore and carnivore. In Herbivores, plants and predators. Proceedings of 38th Symposium of the British Ecological Society, H. Olff, V.K. Brown and R.H. Drent (eds), Blackwell Scientific Publications, in press.
Dicke, M., Sabelis, M.W. and Groeneveld, A. 1986. Vitamin A deficiency modifies response of predatory mite Amblyseius potentillae to volatile kairomone of two-spotted spider mite, Tetranychus urticae. J. Chem. Ecol. 12: 1389–1396.
Dicke, M., Sabelis, M.W. and de Jong, M. 1988. Analysis of prey preference of phytoseiid mites as determined with an olfactometer, predation models and electrophoresis. Exp. Appl. Acarol. 5: 225–241.
Dicke, M., de Jong, M., Alers, M.P.T., Stelder, F.C.T., Wunderink, R. and Post, J. 1989. Quality control of mass-reared arthropods: nutritional effects on performance of predatory mites. J. Appl. Entomol. 108: 462–475.
Dicke, M., van Beek, T.A., Posthumus, M.A., Ben Dom, N., Van Bokhoven, H. and de Groot, A.E. 1990a. Isolation and identification of volatile kairomone that affects acarine predator-prey interactions. Involvement of host plant in its production. J. Chem. Ecol. 16: 381–396.
Dicke, M., Sabelis, M.W., Takabayashi, J., Bruin, J. and Posthumus, M.A. 1990b. Plant strategies of manipulating predator-prey interactions through allelochemicals: prospects for application in pest control. J. Chem. Ecol. 16: 3091–3118.
Dicke, M., Van der Maas, K.J., Takabayashi, J. and Vet, L.E.M. 1990c. Learning affects response to volatile allelochemicals by predatory mites. Proc. Exp. Appl. Entomol. NEV (Amsterdam) 1: 31–36.
Dicke, M., Sabelis, M.W., De Jong, M. and Alers, M.P.T. 1990d. Do phytoseiid mites select the best prey species in terms of reproductive success? Exp. Appl. Acarol. 8: 161–173.
Dicke, M., Dijkman, H. and Wunderink, R. 1991a. Response to synomones as a parameter in quality control of predatory mites. In Proceedings of the Fifth Workshop IOBC Global Working Group ‘Quality Control of Mass Reared Arthropods’, F. Bigler (ed.), pp. 56–65. Wageningen.
Dicke, M., Sabelis, M.W., Bogaers, R.J.F., Alers, M.P.T. and Van Halder, I. 1991b. Kairomone perception by a predatory mite: behavioural analysis of chemoreceptor-carrying extremities. Proc. Exp. Appl. Entomol. NEV (Amsterdam) 2: 179–184.
Dicke, M., Van Baarlen, P., Wessels, R. and Dijkman, H. 1993. Herbivory induces systemic production of plant volatiles that attract predators of the herbivore: extraction of endogenous elicitor. J. Chem. Ecol. 19: 581–599.
Geden, C.J., Smith, L., Long, S.J. and Rutz, D.A. 1992. Rapid deterioration of searching behavior, host destruction, and fecundity of the parasitoid Muscidifurax raptor (Hymenoptera: Pteromalidae) in culture. Ann. Entomol. Soc. Am. 85: 179–187.
Helle W. and Sabelis, M.W. (eds) 1985. Spider Mites. Their Biology, Natural Enemies and Control, Vol. 1a. Elsevier, Amsterdam.
Horton, D.R. and Moore, J. 1993. Behavioral effects of parasites and pathogens in insect hosts. In Parasites and pathogens of insects, Vol. 1, N.E. Beckage, S.N. Thompson and B.A. Federici (eds), pp. 107–124. Academic Press, San Diego.
Huffaker, C.B. 1958. Experimental studies on predation: dispersion factors and predator-prey oscillations. Hilgardia 27: 343–383.
Jagers op Akkerhuis, G., Sabelis, M.W. and Tjallingii, W.F., 1985. Ultrastructure of chemoreceptors on the pepidalps and first tarsi of Phytoseiulus persimilis. Exp. Appl. Acarol. 1: 235–251.
Janssen, A., Hofker, C.D., Braun, A.R., Mesa, N., Sabelis, M.W. and Bellotti, A.C. 1990. Preselecting predatory mites for biological control: the use of an olfactometer. Bull. Entomol. Res. 80: 177–181.
Janssen, A., Van Alphen, J.J.M., Sabelis, M.W. and Bakker, K. 1995. Specificity of odour mediated avoidance of competition in Drosophila parasitoids. Behav. Ecol. Sociobiol. 36: 229–235.
Janssen, A., Bruin, J., Jacobs, G., Schraag, R. and Sabelis, M.W. 1997. Predators use volatiles to avoid prey patches with conspecifics. J. Animal Ecol. 66: 223–232.
Janssen, A., Pallini, A., Venzon, M. and Sabelis, M.W. 1998. Behavioural ecology of food web interactions among plant inhabiting mites and thrips. (submitted).
Koveos, D.S., Kouloussis, N.A. and Broufas, G.D. 1995. Olfactory responses of the predatory mite Amblyseius andersoni Chant (Acari, Phytoseiidae) to bean plants infested by the spider mite Tetranychus urticae Koch (Acari, Tetranychidae). J. Appl. Entomol. 119: 615–619.
Krebs, J.R. and Davies, N.B. (eds) 1991. Behavioural Ecology. An Evolutionary Approach. Blackwell Scientific Publications, Oxford.
Krips, O.E., Willems, P.E.L. and Dicke, M. 1996. Suitability of the ornamental crop Gerbera jamesonii for spider mites and the attraction of predators in response to spider mite damage. Bull. IOBC/WPRS 19(5): 81–87.
Laing, J. E. and Huffaker, C.B. 1969. Comparative studies of predation by Phytoseiulus persimilis Athias-Henriot and Metaseiulus occidentalis (Nesbitt) (Acarina: Phytoseiidae) on populations of Tetranychus urticae Koch (Acarina: Tetranychidae). Res. Popul. Ecol. 11: 105–126.
Loughrin, J.H., Manukian, A., Heath, R.R. and Tumlinson, J.H. 1995. Volatiles emitted by different cotton varieties damaged by feeding beet armyworm larvae. J. Chem. Ecol. 21: 1217–1227.
Overmeer, W.P.J. 1981. Notes on breeding phytoseiid mites from orchards (Acarina: Phytoseiidae) in the laboratory. Med. Fac. Landbouww. Ghent 46: 503–509.
Overmeer, W.P.J. and Van Zon, A.Q. 1983. The effect of different kinds of food on the induction of diapause in the predacious mite Amblyseius potentillae. Entomol. Exp. Appl. 33: 27–30.
Overmeer, W.P.J., Nelis, H.J.C.F., de Leenheer, A.P., Calis, J.N.M. and Veerman, A., 1989. Effect of diet on the photoperiodic introduction of diapause in three species of predatory mite, Amblyseius potentillae, A. cucumeris and Typhlodromus pyri. Exp. Appl. Acarol. 7: 281–287.
Poulin, R., Brodeur, J. and Moore, J. 1994. Parasite manipulation of host behaviour: should hosts always lose? Oikos 70: 479–484.
Robertson, I.C., Roitberg, B.D., Williamson, I. and Senger, S. 1995. Contextual chemical ecology: an evolutionary approach to the chemical ecology of insects. Am. Entomol. 41: 237–239.
Roitberg, B.D. 1992. Why an evolutionary perspective?. In Insect chemical ecology. An evolutionary approach, B.D. Roitberg and M.B. Isman (eds), pp. 5–19. Chapman & Hall, New York.
Sabelis, M.W. 1981. Biological Control of Two-spotted Spider Mites Using Phytoseiid Predators. Part I: Modelling the Predator-Prey Interaction at the Individual Level. Centre for Agricultural Publishing and Documentation, PUDOC, Wageningen.
Sabelis, M.W. and Afman, B.P. 1994. Synomone-induced suppression of take-off in the phytoseiid mite Phytoseiulus persimilis Athias-Henriot. Exp. Appl. Acarol. 18: 711–721.
Sabelis, M.W. and Dicke, M. 1985. Long-range dispersal and searching behaviour. In Spider mites. Their biology, natural enemies and control, Vol. 1b, W. Helle and M.W. Sabelis (eds), pp. 141–160. Elsevier, Amsterdam.
Sabelis, M.W. and Van de Baan, H.E. 1983. Location of distant spider mite colonies by phytoseiid predators: demonstration of specific kairomones emitted by Tetranychus urticae and Panonychus ulmi. Entomol. Exp. Appl. 33: 303–314.
Sabelis, M.W. and Van der Meer, J. 1986. Local dynamics of the interaction between predatory mites and two-spotted spider mites. In Dynamics of physiologically structured populations, J.A.J. Metz and O. Diekman (eds), pp. 322–343. Springer, Berlin.
Sabelis, M.W. and Van der Weel, J.J. 1993. Anemotactic responses of the predatory mite, Phytoseiulus persimilis Athias-Henriot, and their role in prey finding. Exp. Appl. Acarol. 17: 521–529.
Sabelis, M.W., Van Alebeek, F., Bal, A., van Bilsen, J., Van Heijningen, T., Kaizer, P., Kramer, G., Snellen, H., Veenenbos, R. and Vogelezang, J. 1983. Experimental validation of a simulation model of the interaction between Phytoseiulus persimilis and Tetranychus urticae on cucumber. Bull. IOBC/WPRS 6(3): 207–229.
Sabelis, M.W., Afman, B.P. and Slim, P.J. 1984a. Location of distant spider mite colonies by Phytoseiulus persimilis: localization and extraction of a kairomone. Acarology VI, Vol. 1: 431–440.
Sabelis, M.W., Vermaat, J.E. and Groeneveld, A. 1984b. Arrestment responses of the predatory mite, Phytoseiulus persimilis, to steep odour gradients of a kairomone. Physiol. Entomol. 9: 437–446.
Schütte, C., Hulshof, J., Dijkman, H. and Dicke, M. 1995. Change in foraging behaviour of the predatory mite Phytoseiulus persimilis: some characteristics of a mite population that does not respond to herbivore-induced synomones. Proc. Exp. Appl. Entomol. NEV (Amsterdam) 6: 133–139.
Schütte, C., Van Baarlen, P., Dijkman, H. and Dicke, M. 1996. How can predatory mites lose their response to plant signals? Proc. Exp. Appl. Entomol. NEV (Amsterdam) 7: 195–196.
Shimoda, T., Takabayashi, J., Ashihara, W. and Takafuji, A. 1997. Response of the predatory insect Scolothrips takahashii toward herbivore-induced plant synomone under both laboratory and field conditions. J. Chem. Ecol. 23: 2033–2048.
Stephens, D.W. and Krebs, J.R. 1986. Foraging Theory. Princeton University Press. Princeton.
Takabayashi, J. and Dicke, M. 1996. Plant-carnivore mutualism through herbivore-induced carnivore attractants. Trends Plant Sci. 1: 109–113.
Takabayashi, J., Dicke, M. and Posthumus, M.A. 1991. Variation in composition of predator-attracting allelochemicals emitted by herbivore-infested plants: relative influence of plant and herbivore. Chemoecology 2: 1–6.
Takabayashi, J., Dicke, M., Takahashi, S., Posthumus, M.A. and van Beek, T.A. 1994a. Leaf age affects composition of herbivore-induced synomones and attraction of predatory mites. Journal of Chemical Ecology 20: 373–386.
Takabayashi, J., Dicke, M. and Posthumus, M.A. 1994b. Volatile herbivore-induced terpenoids in plant-mite interactions: variation caused by biotic and abiotic factors. J. Chem. Ecol. 20: 1329–1354.
Tumlinson, J.H., Turlings, T.C.J. and Lewis, W.J. 1992. The semiochemical complexes that mediate insect parasitoid foraging. Agricult. Zool. Rev. 5: 221–252.
Turlings, T.C.J. and Tumlinson, J.H. 1992. Systemic release of chemical signals by herbivore-injured corn. Proc. Natl Acad. Sci. USA 89: 8399–8402.
Turlings, T.C.J., Wäckers, F.L., Vet, L.E.M., Lewis, W.J. and Tumlinson, J.H. 1993. Learning of host-finding cues by Hymenopterous parasitoids. In Insect learning, D.R. Papaj and A.C. Lewis (eds), pp. 51–78. Chapman & Hall, New York.
Turlings, T.C.J., Loughrin, J.H., McCall, P.J., Röse, U.S.R., Lewis, W.J. and Tumlinson, J.H. 1995. How caterpillar-damaged plants protect themselves by attracting parasitic wasps. Proc. Natl Acad. Sci. USA 92: 4169–4174.
Van Houten, Y.M., Overmeer, W.P.J. and Veerman, A. 1987. Thermoperiodically induced diapause in a mite in constant darkness is vitamin A dependent. Experientia 43: 933–935.
Van Zon, A.Q., Overmeer, W.P.J. and Veerman, A. 1981. Carotenoids function in photoperiodic induction of diapause in a predacious mite. Science 213: 1131–1133.
Veerman, A. 1992. Diapause in phytoseiid mites: a review. Exp. Appl. Acarol. 14: 1–60
Vet, L.E.M. and Dicke, M. 1992. Ecology of infochemical use by natural enemies in a tritrophic context. Annu. Rev. Entomol. 37: 141–172.
Vet, L.E.M., Wäckers, F.L. and Dicke, M. 1991. How to hunt for hiding hosts: the reliability—detectability problem in foraging parasitoids. Neth. J. Zool. 41: 202–213.
Vet, L.E.M., Lewis, W.J. and Carde, R.T. 1995. Parasitoid foraging and learning. In Chemical ecology of insects 2, R.T Carde and W.J. Bell (eds), pp. 65–101. Chapman & Hall, New York.
Visser, M.E., Van Alphen, J.J.M. and Nell, H.W. 1990. Adaptive superparasitism and patch time allocation in solitary parasitoids: the influence of the number of parasitoids depleting a patch. Behaviour 114: 21–36.
Walde, S.J. 1995. Internal dynamics and metapopulations: experimental tests with predator-prey systems. In Population dynamics. New approaches and synthesis, N. Cappuccino and P.W. Price (eds), pp. 173–193. Academic Press, San Diego.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Dicke, M., Takabayashi, J., Posthumus, M.A. et al. Plant—Phytoseiid Interactions Mediated by Herbivore-Induced Plant Volatiles: Variation in Production of Cues and in Responses of Predatory Mites. Exp Appl Acarol 22, 311–333 (1998). https://doi.org/10.1023/A:1024528507803
Issue Date:
DOI: https://doi.org/10.1023/A:1024528507803