Abstract
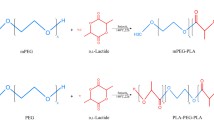
Purpose. The pure antiestrogen RU58668 (RU) was encapsulated within nanospheres (NS) and nanocapsules (NC) prepared from different polyester copolymers with poly(ethylene glycol) (PEG) chains. The influence of their physicochemical properties on drug release in vitro and their susceptibility to opsonization were evaluated.
Methods. RU-loaded PEG-bearing nanoparticles (NP) prepared by interfacial deposition of preformed polymer were characterized (size, zeta potential, percentage encapsulation and loading). In vitro release kinetics were studied in the presence of 10% fetal calf serum (FCS). Their opsonization in mouse serum was evaluated by silver staining of SDS-PAGE and Western blotting of desorbed proteins.
Results. The NS were smaller than NC and had a zeta potential close to zero and a higher percentage of loading. RU release from NS in vitro was reduced as compared with the dissolution profile of free RU in a serum-containing medium. Decreased opsonin adsorption at the surface of pegylated NS was observed.
Conclusion. Small nanoparticulate systems containing a high load of pure antiestrogen, showing reduced drug release, have been developed. Among the six nanosphere preparations containing RU, two show a size below 200 nm, and two others undergo reduced protein adsorption in the presence of serum, compatible with increased persistence in the blood.
Similar content being viewed by others
REFERENCES
J. I. MacGregor and V. C. Jordan. Basic guide to the mechanisms of anti-estrogen action. Pharmacol. Rev. 50:151–196 (1998).
P. Van de Velde, F. Nique, F. Bouchoux, J. Bremaud, M. C. Hameau, D. Lucas, C. Moratille, S. Viet, D. Philibert, and G. Teutsch. RU 58,668, a new pure anti-estrogen inducing a regression of human mammary carcinoma implanted in nude mice. J. Steroid Biochem. Mol. Biol. 48:187–196 (1994).
P. Van de Velde, F. Nique, J. Bremaud, M. C. Hameau, D. Philibert, and G. Teutsch. Exploration of the therapeutic potential of the anti-estrogen RU 58668 in breast cancer treatment. Ann. NY Acad. Sci. 761:164–175 (1995).
P. Van de Velde, F. Nique, P. Planchon, G. Prevost, J. Bremaud, M. C. Hameau, V. Magnien, D. Philibert, and G. Teutsch. RU 58668: further in vitro and in vivo pharmacological data related to its antitumoral activity. J. Steroid Biochem. Mol. Biol. 59:449–457 (1996).
S. M. Moghimi, A. C. Hunter, and J. C. Murray. Long-circulating and target-specific nanoparticles: theory to practice. Pharmacol. Rev. 53:283–318 (2001).
M. Vittaz, D. Bazile, G. Spenlehauer, T. Verrecchia, M. Veillard, F. Puisieux, and D. Labarre. Effect of PEO surface density on long-circulating PLA–PEO nanoparticles which are very low complement activators. Biomaterials 17:1575–1581 (1996).
A. Gabizon and F. Martin. Polyethylene glycol-coated (pegylated) liposomal doxorubicin. Rationale for use in solid tumours. Drugs 54:15–21 (1997).
P. Quellec, R. Gref, L. Perrin, E. Dellacherie, F. Sommer, J. M. Verbavatz, and M. J. Alonso. Protein encapsulation within polyethylene glycol-coated nanospheres. I. Physicochemical characterization. J. Biomed. Mater. Res. 42:45–54 (1998).
R. Gref, Y. Minamitake, M. T. Peracchia, V. Trubetskoy, V. Torchilin, and R. Langer. Biodegradable long-circulating polymeric nanospheres. Science 263:1600–1603 (1994).
R. Gref, A. Domb, P. Quellec, T. Blunk, R. H. Müller, J. M. Verbavatz, and R. Langer. The controlled intravenous delivery of drugs using PEG-coated sterically stabilized nanospheres. Adv. Drug Deliv. Rev. 16:215–233 (1995).
H. Fessi, F. Puisieux, J. Devissaguet, N. Ammoury, and S. Benita. Nanocapsules formation by interfacial polymer deposition following solvent displacement. Int. J. Pharm. 55:r1-r4 (1989).
W. Schaffner and C. Weissmann. A rapid, sensitive, and specific method for the determination of protein in dilute solution. Anal. Biochem. 56:502–514 (1973).
V. C. Mosqueira, P. Legrand, A. Gulik, O. Bourdon, R. Gref, D. Labarre, and G. Barratt. Relationship between complement activation, cellular uptake and surface physicochemical aspects of novel PEG-modified nanocapsules. Biomaterials 22:2967–2979 (2001).
S. K. Hobbs, W. L. Monsky, F. Yuan, W. G. Roberts, L. Griffith, V. P. Torchilin, and R. K. Jain. Regulation of transport pathways in tumor vessels: role of tumor type and microenvironment. Proc. Natl. Acad. Sci. USA 95:4607–4612 (1998).
A. Nagayasu, K. Uchiyama, and H. Kiwada. The size of liposomes: a factor which affects their targeting efficiency to tumors and therapeutic activity of liposomal antitumor drugs. Adv. Drug Deliv. Rev. 40:75–87 (1999).
M. Leroueil-Le Verger, L. Fluckiger, Y. I. Kim, M. Hoffman, and P. Maincent. Preparation and characterization of nanoparticles containing an antihypertensive agent. Eur. J. Pharm. Biopharm. 46:137–143 (1998).
Y. Li, Y. Pei, X. Zhang, Z. Gu, Z. Zhou, W. Yuan, J. Zhou, J. Zhu and X. Gao. PEGylated PLGA nanoparticles as protein carriers: synthesis, preparation and biodistribution in rats. J. Control. Release 71:203–211 (2001).
K. Avgoustakis, A. Beletsi, Z. Panagi, P. Klepetsanis, A. G. Karydas' and D. S. Ithakissios. PLGA-mPEG nanoparticles of cisplatin: in vitro nanoparticle degradation, in vitro drug release and in vivo drug residence in blood properties. J. Control. Release 79:123–135 (2002).
T. Govender, T. Riley, T. Ehtezazi, M. C. Garnett, S. Stolnik, L. Illum, and S. S. Davis. Defining the drug incorporation properties of PLA-PEG nanoparticles. Int. J. Pharm. 199:95–110 (2000).
I. Brigger, P. Chaminade, V. Marsaud, M. Appel, M. Besnard, R. Gurny, M. Renoir, and P. Couvreur. Tamoxifen encapsulation within polyethylene glycol-coated nanospheres. A new anti-estrogen formulation. Int. J. Pharm. 214:37–42 (2001).
J. Matsumoto, Y. Nakada, K. Sakurai, T. Nakamura, and Y. Takahashi. Preparation of nanoparticles consisted of poly(L-lactide)–poly(ethylene glycol)–poly(L-lactide) and their evaluation in vitro. Int. J. Pharm. 185:93–101 (1999).
R. Gref, P. Quellec, A. Sanchez, P. Calvo, E. Dellacherie, and M. J. Alonso. Development and characterization of CyA-loaded poly(lactic acid)–poly(ethylene glycol)PEG micro-and nanoparticles. Comparison with conventional PLA particulate carriers. Eur. J. Pharm. Biopharm. 51:111–118 (2001).
H. M. Redhead, S. S. Davis, and L. Illum. Drug delivery in poly(lactide–co-glycolide) nanoparticles surface modified with poloxamer 407 and poloxamine 908: in vitro characterisation and in vivo evaluation. J. Control. Release 70:353–363 (2001).
V. Mosqueira, P. Legrand, R. Gref, and G. Barratt. In-vitro release kinetic studies of PEG-modified nanocapsules and nanospheres loaded with a lipophilic drug: halofantrine base. Proc. 26 Int. Symp. Control. Release Bioactive Mater. 26:1074–1075 (1999).
M. Polakovic, T. Gorner, R. Gref, and E. Dellacherie. Lidocaine loaded biodegradable nanospheres. II. Modelling of drug release. J. Control. Release 60:169–177 (1999).
A. L. Klibanov, K. Maruyama, V. P. Torchilin, and L. Huang. Amphipathic polyethyleneglycols effectively prolong the circulation time of liposomes. FEBS Lett. 268:235–237 (1990).
R. Gref, M. Luck, P. Quellec, M. Marchand, E. Dellacherie, S. Harnisch, T. Blunk, and R. H. Muller. "Stealth" corona-core nanoparticles surface modified by polyethylene glycol (PEG): influences of the corona (PEG chain length and surface density) and of the core composition on phagocytic uptake and plasma protein adsorption. Colloids Surf. B Biointerfaces 18:301–313 (2000).
U. Gaur, S. K. Sahoo, T. K. De, P. C. Ghosh, A. Maitra, and P. K. Ghosh. Biodistribution of fluoresceinated dextran using novel nanoparticles evading reticuloendothelial system. Int. J. Pharm. 202:1–10 (2000).
K. Ogawara, K. Furumoto, Y. Takakura, M. Hashida, K. Higaki, and T. Kimura. Surface hydrophobicity of particles is not necessarily the most important determinant in their in vivo disposition after intravenous administration in rats. J. Control. Release 77:191–198 (2001).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ameller, T., Marsaud, V., Legrand, P. et al. Polyester-Poly(Ethylene Glycol) Nanoparticles Loaded with the Pure Antiestrogen RU 58668: Physicochemical and Opsonization Properties. Pharm Res 20, 1063–1070 (2003). https://doi.org/10.1023/A:1024418524688
Issue Date:
DOI: https://doi.org/10.1023/A:1024418524688