Abstract
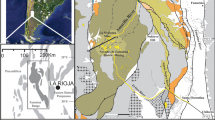
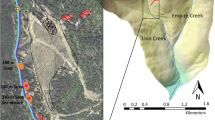
The spatial variability in chemical composition of water and sediments along Snow Fork, a stream draining 70 km2 of southeastern Ohio, was investigated under low-flow conditions. The stream is affected by acid mine drainage (AMD) beginning atEssex Mine, an abandoned mine opening, and extending 23 km downstream to the confluence with Monday Creek. Volumetric discharge and changes in stream water and sediment metalconcentrations were examined to identify chemical interactionsand processes controlling the transport and fate of metalcontaminants. The stream loses water to the groundwater system insome sections. The water loss probably occurs through fracturesconnecting the stream to underlying underground coal mines. Massbalance (loading) and mineral saturation index calculations wereused to identify metal sources and sinks. Dissolved metal loadingincreases downstream along the length of Snow Fork, despite theprecipitation of metals as hydroxides in the streambed,indicating multiple groundwater sources of AMD along the flowpath. Relatively high dissolved metal concentrations and lowsediment metal concentrations occur where the pH is low,indicating that local sediment-water interaction dominates masstransfer between sediments and water. Calculated mineralsaturation indexes indicate that aluminum and iron hydroxidesprecipitate in some stream segments and dissolve in others. X-raydiffractograms of sediments show two distinct mineral groups.Amorphous or weakly crystalline minerals dominate one group foundnear the stream headwaters near the underground mine. Crystallinemineral phases dominate the sediments downstream. Thesediffractograms contain the primary peaks for quartz, kaoliniteand illite all of which constitute the local sandstones, shalesor underclay. Peaks of amorphous phases of iron and manganese,if present, are obscured. The implications of these findings arethat the transport of metals in sediments may be as important asdissolved metal transport in estimating the overall stream load,particularly if downstream sources of AMD may remobilize metalsfrom soluble precipitates.
Similar content being viewed by others
References
Bencala, K. E.: 1984, ‘Interactions of solutes and streambed sediment’, Water Res. Res. 20, pp. 1797-1803.
Bencala, K. E., McKnight, D. M. and Zellweger, G. W.: 1987, ‘Evaluation of natural tracers in an acidic and metal-rich stream’, Water Res. Res. 23, pp. 827-836.
Bencala, K. E., McKnight, D. M. and Zellweger, G. W.: 1990, ‘Characterization of transport in an acidic and metal-rich mountain stream based on a lithium tracer injection and simulations of transient storage’, Water Res. Res. 26, 989-1000.
Bigham, J. M., Schwertmann, U. and Pfab, G.: 1996, ‘Influence of pH on mineral speciation in a bioreactor simulating acid mine drainage’, Appl. Geochem. 11, 845-849.
Botoman, G. and Stith, D. A.: 1978, ‘Analysis of Ohio coals’, Ohio Geolog. Surv. Inform. Circ. 47, 148 pp.
Broshears, R. E., Runkel, R. L., Kimball, B. A. and Bencala, K. E.: 1996, ‘Reactive solute transport in an acidic stream: experimental pH increase and simulation of controls on pH, aluminum, and iron’, Env. Sci. Techn. 30, 3016-3024.
Carter, M. R.: 1993, Soil Sampling and Methods of Analysis, Lewis Publishers, Ann Arbor,Michigan, pp. 461-462.
Carver, R. E.: 1971, Procedures in Sedimentary Petrology, Wiley Interscience, New York, pp. 531- 569.
Chapman, B. M.: 1982, ‘Numerical simulation of the transport and speciation of nonconservative chemical reactants in rivers’, Water Res. Res. 18, 155-167.
Crowell, C. S.: 1979, ‘The Mineralogic Analysis of the Underclays of the Lower Allegheny Formation in Jackson, Vinton, Hocking, and Perry Counties, Ohio’, M.S. Thesis, Miami University, Ohio, 130 pp.
Drever, J. I.: 1997, The Geochemistry of Natural Waters, 3rd Ed., Prentice Hall, New Jersey, 437 pp.
Durkin, T. V. and Herrmann, J. G.: 1996, Managing Environmental Problems at Inactive and Abandoned Metal Mine Sites, EPA Seminar Publication. EPA/625/R-95/007, pp. 1-3.
Dzombak, D. A. and Morel, F.M.M.: 1990, Surface Complexation Modeling Hydrous Ferric Oxides, John Wiley and Sons, New York, 393 pp.
Friel, E. A., Ehlke, T. A., Hobba, W. A., Jr., Ward, S. M. and Shultz, R. A.: 1984, Hydrology of Area 8, Eastern Coal Province, West Virginia and Ohio, United States Geological Survey Open File Report; 84-463, 78 pp.
Fuge R., Pearce, F. M., Pearce, N. J. G. and Perkins, W. T.: 1994, ‘Acid Mine Drainage in Wales and Influence of Ochre Precipitation on Water Chemistry’, in C. N. Alpers and D. W. Blowes (eds), Environmental Geochemistry of Sulfide Oxidation, ACS Symposium Series 550, pp. 261-274.
HACH Company: 1997, Water Analysis Handbook, 3rd Ed., 1309 pp.
Hartke, E. J.: 1974, ‘Source of Acid Mine Drainage in the Monday Creek Drainage Basin, Southeastern Ohio’, M.S. Thesis, Geological Sciences, Ohio University, Ohio, 134 pp.
Herr, C. and Gray, N. F.: 1996, Water, Science, and Technology 33, pp. 255-261.
Herschy, R. W.: 1985, Streamflow Measurement, Elsevier Applied Science Publishers, London, 91 pp.
JCPDS: 1989, Powder Diffraction File Inorganic Phases, International Centre for Diffraction Data, pp. 719-789.
Johnson, C. A.: 1986, ‘The regulation of trace element concentrations in river and estuarine waters contaminated with acid mine drainage: The adsorption of Cu and Zn on amouphous Fe oxyhydroxides’, Geochim. Cosmochim. Acta 50, 2433-2438.
Kimball, B. A., Broshears, R. E., Bencala, K. E. and McKnight, D.M.: 1994a, ‘Coupling of hydrologic transport and chemical reactions in a stream affected by acid mine drainage’, Env. Sci. Techn. 28, 2065-2073.
Kimball, B. A., Broshears, R. E. McKnight, D. M. and Bencala, K. E.: 1994b, ‘Effects of Instream pH Modification on Transport of Sulfide-Oxidation Products’, in C. N. Alpers and D. W. Blowes (eds), Environmental Geochemistry of Sulfide Oxidation, ACS Symposium Series 550, pp. 224-243.
Kimball, B. A., Callender, E. and Axtmann, E. V.: 1995, ‘Effects of colloids on metal transport in a river receiving acid mine drainage, upper Arkansas River, Colorado, U.S.A.’, Appl. Geochem. 10, 285-306.
Klein, C. and Hurlbut, C. S., Jr.: 1993, Manual of Mineralogy, 21st Ed., John Wiley and Sons, Inc., New York, 681 pp.
Kleinmann, R. L. P., Allwes, R. A. Jones, P. A. Matertic, R. J. and Statnick, R.: 1995, ‘Environmental issues of the Appalachian coal region’, Min. Engin. 4, 1120-1123.
Levy, D. B., Custis, K. H., Casey, W. H. and Rock, P. A.: 1997, ‘A comparison of metal attenuation in mine residue and overburden material from an abandoned copper mine’, Appl. Geochem. 12, 203-211.
López, D. L., Overly, B., Robbins, E. I. and Carroll, K.: 1999, ‘The Role of Flow Regime on the Chemical Evolution of AcidicWaters Discharged from an Abandoned Underground Coal Mine’, in Proc. Sudbury,99, Mining in the Environ', pp. 89-98.
Mancl, K. and Rector, D.: 2001, ‘Reuse of Reclaimed Waste Water through irrigation for Ohio Communities’, Extension Bulletin 860, The Ohio State University, http://www.ag.ohiostate. edu/?ohioline/b860/
McKnight, D. M., Kimball, B. A. and Bencala, K. E.: 1988, ‘Iron photoreduction and oxidation in an acidic mountain stream’, Science 240, 637-640.
McKnight, D. M. and Bencala, K. E.: 1989, ‘Reactive iron transport in an acidic mountain stream in Summit County, Colorado: A hydrologic perspective’, Geochim. Cosmochim. Acta 53, 2225- 2234.
Nagano, T., Nakashima, S., Nakayama, S., Osaka, K. and Senoo, M.: 1992, ‘Color variations associated with rapid formation of goethite from proto-ferrihydrite at pH 13 and 40 ?C’, Clays and Clay Minerals 4, 600-607.
Nordstrom, D. K.: 1977, ‘Hydrogeochemical andMicrobiological Factors Affecting the HeavyMetal Chemistry of an Acid Mine Drainage System’, Ph.D. Dissertation, Stanford University, CA, 210 pp.
Nordstrom, D. K. and Ball, J.W.: 1986, ‘;The geochemical behavior of aluminum in acidified surface waters’, Science 232, 54-56.
Parkhurst, D. L.: 1995, User's Guild to PHREEQC-A Computer Program for Speciation, Reaction-Path, Advective-Transport, and Inverse Geochemical Calculations, U.S. Geologic Survey Water-Resources Investigations Report 95-4227, 143 pp.
Pigati, E. M. and López, D. L.: 1999, ‘Effect of subsidence on recharge at abandoned coal mines generating acidic drainage: the majestic mine, Athens County, Ohio’, Mine Water Env. 18, 45-66.
Ritchie, A. I. M.: 1994, ‘Rates of Mechanisms that Govern Pollutant Generation from PyriticWastes’, in C. N. Alpers and D.W. Blowes (eds), Environmental Geochemistry of Sulfide Oxidation, ACS Symposium Series 550, pp. 224-243.
Runkel, R. L., McKnight, D. M. and Bencala, K. E.: 1996, ‘Reactive solute transport in streams 2. Simulation of a pH modification experiment’, Water Res. Res. 32, 419-430.
Singer P. C. and Stumm, W.: 1970, ‘Acidic mine drainage: the rate-determining step’, Science 167, 1121-1123.
Shimala, J. and Borch, M. A.: 1999, Monday Creek Watershed Acid Mine Drainage Abatament and Treatment Plan II, Unpublished report for USDI Office of Surface Mining, Ohio Department of Natural Resources, Division of Mines and Reclamation, and the USDA Natural Resources Conservation Service, 83 pp.
Stachler, P. M.: 1997, ‘Chemical and Hydrologic Variability of Acid Mine Drainage From Abandoned Esco #40 Underground Coal Mine, Ohio’, M. S. Thesis, Geological Sciences, Ohio University, Ohio, 103 pp.
Steefel, C. I. and Lasaga, A. C.: 1992, ‘Putting transport into water-rock interaction models’, Geology 20, 680-684.
Sturgeon, M. T. and Associates: 1958, The Geology and Mineral Resources of Athens County, Ohio, Ohio Geologic Survey Bulletin 57, 48-92.
Symader, W., Schorer, M. and Bierl, R.: 1994, ‘Temporal Variations of Environmental Pollutants in Channel Sediments’, in Variability in Stream Erosion and Sediment Transport, Proceedings of the Canberra Symposium, IAHS Publ. No. 224, pp. 491-498.
Webster J. G., Nordstrom, D. K. and Smith, K. S.: 1994, ‘Transport and Natural Attenuation of Cu, Zn, As, and Fe in the Acid Mine Drainage of Leviathan and Bryant Creeks’, in C. N. Alpers and D. W. Blowes (eds), Environmental Geochemistry of Sulfide Oxidation, ACS Symposium Series 550, pp. 299-321.
Worsley, S. J.: 1996, ‘Flooding, Stream Competence, and Bedload Transport in Snow Fork of Monday Creek, Athens and Hocking Counties, Ohio’, M. S. Thesis, Ohio University, Ohio, 109 pp.
U.S. Geological Survey: 1995a, 7.5 Minute Nelsonville Quadrangle Topographic Map, Reston.
U.S. Geological Survey: 1995b, 7.5 Minute New Straitsville Quadrangle Topographic Map, Reston.
USEPA: 1986, Sampling Preparation Methods, Method 3050, Acid Digestion of Sediments, Sludges, and Soils, Government Printing Office, 4 pp.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Carroll, K.C., López, D.L. & Stoertz, M.W. Solute Transport at Low Flow in an Acid Stream in Appalachian Ohio. Water, Air, & Soil Pollution 144, 195–222 (2003). https://doi.org/10.1023/A:1022925519292
Issue Date:
DOI: https://doi.org/10.1023/A:1022925519292