Abstract
Purpose. The purpose of this work was to demonstrate the feasibility of using near-infrared spectroscopy (NIRS) to monitor the freeze-drying process in-situ.
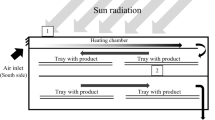
Methods. The experiment was performed in a pilot-scale freeze-dryer, in which the NIRS probe was interfaced using a lead-through to the lyophilizer. Special equipment for the sample presentation was developed. NIRS measurements were made using a FT (Fourier transform)-NIR spectrometer fitted with a single fiber reflectance probe.
Results. The physical changes, that is, freezing, sublimation, and desorption, generated significant spectral changes. There was good agreement between NIRS monitoring and product temperature monitoring about the freezing process and the transition from frozen solution to ice-free material. The NIRS monitoring also provided new information about the process that was not possible to detect with product temperature monitoring, such as the rate of the desorption process and the steady-state where the drying was complete. The NIRS monitoring yields significantly more information about the actual process and essentially explains the observed changes of the product temperature during the lyophilization process.
Conclusions. NIRS monitoring is a viable tool for in-situ monitoring, both qualitatively and quantitatively. It can facilitate investigations of the drying process within a sample. The small volume monitored makes sample presentation very important.
Similar content being viewed by others
REFERENCES
J. Dalgleish. Freeze-drying for the food industries. Elsevier Science Publishers, London, 1990.
A. T. P. Skrabanja, A. L. J. De Meere, R. A. De Ruiter, and P. J. M. Van den Oetelaar. Lyophilization of biotechnology products. PDA J. Pharm. Sci. Technol. 48:311-317 (1994).
V. Rindler, S. Luneberger, P. Schwindke, I. Heschel, and G. Rau. Freeze-drying of red blood cells at ultra-Low temperatures. Cryobiology 38:2-15 (1999).
W. Wang. Lyophilization and development of solid protein pharmaceuticals. Int. J. Pharm. 203:1-60 (2000).
P. Cameron. Good Pharmaceutical Freeze-Drying Practice, Interpharm Press, Inc., Buffalo Grove, 1997.
H. Seager. Drug-delivery products and the Zydis fast-dissolving dosage form. J. Pharm. Pharmacol. 50:375-382 (1998).
M. Diestelhorst, S. Grunthal, and R. Suverkrup. Dry drops: a new preservative-free drug delivery system. Graefes Arch. Clin. Exp. Ophthalmol. 237:394-398 (1999).
S. L. Nail and W. Johnson. Methodology for in-process determination of residual water in freeze-dried products. International Symposium on Biological Product Freeze-Drying and Formulation Bethesda, USA, 1990. Dev. Biol. Standard 74:137-151 (1991).
N. Milton, M. J. Pikal, M. L. Roy, and S. L. Nail. Evaluation of manometric temperature measurement as a method of monitoring product temperature during lyophilization. PDA J. Pharm. Sci. Technol. 51:7-16 (1997).
A. Bardat, J. Biguet, E. Chatenet, and F. Courteille. Moisture measurement: A new method for monitoring freeze-drying cycles. J. Parenter. Sci. Technol. 47:293-299 (1993).
N. Genin, F. RenÉe, and G. Corrieu. A method for on-line determination of residual water content and sublimation end-point during freeze drying. Chem. Eng. Process. 35:255-263 (1996).
J. P. Connelly and J. V. Welch. Monitor lyophilization with mass spectrometer gas analysis. J. Parenter. Sci. Technol. 47:70-75 (1993).
R. K. Cavatur and R. Suryanarayanan. Characterization of phase transitions during freeze-drying by in situ X-ray powder diffractometry. Pharm. Dev. Technol. 3:579-586 (1998).
M. J. P. Marques, L. C. Loch, E. Wolff, and D. N. Rutledge. Monitoring freeze drying by low resolution pulse NMR: Determination of sublimation endpoint. J. Food Sci. 56:1707-1713 (1991).
R. L. Remmele, C. Stushnoff, and J. F. Carpenter. Real-time in situ monitoring of lysozyme during lyophilization using infrared spectroscopy-dehydration stress in the presence of sucrose. Pharm. Res. 14:1548-1555 (1997).
A. P. Mackenzie. Apparatus for microscopic observations during freeze-drying. Biodynamica 9:213-222 (1964).
R. De Maesschalck, F. Cuesta SÁnchez, D. L. Massart, P. Doherty, and P. Hailey. On-line monitoring of powder blending with near-infrared spectroscopy. Appl. Spectrosc. 52:725-731 (1998).
O. Berntsson, L.-G. Danielsson, B. Lagerholm, and S. Folestad. Quantitative in-line monitoring of powder blending by near infrared reflection spectroscopy. Powder Technol. 123:185-193 (2002).
P. Frake, D. Greenhalgh, S. M. Grierson, J. M. Hempenstall, and D. R. Rudd. Process control and end-point determination of a fluid bed granulation by application of near infra-red spectroscopy. Int. J. Pharm. 151:75-80 (1997).
J. Rantanen, E. RÄsÄanen, O. Antikainen, and J.-P. Mannermaa. and J. Yliruusi. In-line moisture measurement during granulation with a four-wavelength near-infrared sensor: an evaluation of process-related variables and a development of non-linear calibration model. Chemom. Intel. Lab. Syst. 56:51-58 (2001).
G. Radtke, K. Knop, and C. Lippold. In-process control of direct pelletisation in the rotary fluidised bed using NIR spectroscopy. NIR News 10:4,5,12 (1999).
M. Andersson, M. Josefson, F. Langkilde, and K.-G. Wahlund. Monitoring of a film coating process for tablets using near infrared reflectance spectrometry. J. Pharm. Biomed. Anal. 20:27-37 (1999).
M. Andersson, S. Folestad, J. Gottfries, M. O. Johansson, M. Josefson, and K.-G. Wahlund. Quantitative analysis of film coating in a fluidized bed process by in-line NIR spectrometry and multivariate batch calibration. Anal. Chem. 72:2099-2108 (2000).
J. D. Kirsch and J. K. Drennen. Determination of Film-coated Tablet Parameters by Near-infrared Spectroscopy. J. Pharm. Biomed. Anal. 13:1273-1281 (1995).
J. D. Kirsch and J. K. Drennen. Near-infrared spectroscopic monitoring of the film coating process. Pharm. Res. 13:234-237 (1996).
M. S. Kamat, R. A. Lodder, and P. P. DeLuca. Near-infrared spectroscopic determination of residual moisture in lyophilized sucrose through intact glass vials. Pharm. Res. 6:961-965 (1989).
J. A. Jones, I. R. Last, B. F. MacDonald, and K. A. Prebble. Development and transferability of near-infrared methods for determination of moisture in a freeze-dried injection product. J. Pharm. Biomed. Anal. 11:1227-1231 (1993).
S. Wold, K. Esbensen, and P. Geladi. Principal component analysis. Chemom. Intel. Lab. Syst. 2:37-52 (1987).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Brülls, M., Folestad, S., Sparén, A. et al. In-Situ Near-Infrared Spectroscopy Monitoring of the Lyophilization Process. Pharm Res 20, 494–499 (2003). https://doi.org/10.1023/A:1022680810474
Issue Date:
DOI: https://doi.org/10.1023/A:1022680810474