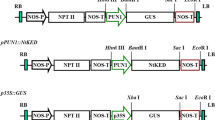
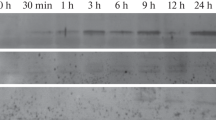
Abstract
The major protein secreted into the nectar of tobacco plants (Nectarin I) is a germin-like protein that has superoxide dismutase activity. We have isolated the gene encoding Nectarin I (NECI) and analyzed the expression of a chloramphenicol acetyl transferase (CAT) marker gene driven by its promoter in transgenic plants. Transgenic plant lines that expressed the CAT gene under control of the full-length NEC1 promoter showed high levels of CAT expression in mature floral nectaries. Tissue specificity of NECI-CAT expression demonstrated that the construct was expressed uniquely in nectaries with a small level of expression in ovary. Further, analysis of its temporal expression showed that the construct is expressed uniquely during those times when nectar is actively being secreted from flowers. An examination of the transcription start site verified that the initiation site of the NECI-CAT mRNA in transgenic plants is identical with that of the native gene in vivo. Two promoter deletion constructs were also prepared and analyzed. Analysis in transgenic plants revealed that the nectary-specific expression is the result of multiple promoter elements and suggests that nectar secretion and flower opening may be coordinately regulated.
Similar content being viewed by others
References
An, G, Ebert, PR, Yi, B-Y, Choi, C-H: Both TATA box and upstream regions are required for nopaline synthase promotor activity in transformed tobacco cells. Mol. Gen. Genet. 203: 245–250 (1986).
An, G, Thornburg, RW, Johnson, R, Hall, G, Ryan, CA: A possible role for 3′ sequences of the wound-inducible potato proteinase inhibitor II K gene in regulating gene expression. In: von Wettstein, D, Chua, N-H (eds) NATO ASI Series A: Life Sciences. Plenum Press, New York (1987).
Ausubel, FM, Brent, R, Kingston, RE, Moore, DD, Seidman, JG, Smith, JA, Struhl, K: Short Protocols in Molecular Biology. John Wiley & Sons, New York (1992).
Baker, HG, Baker, I: Amino acids in nectar and their evolutionary significance. Nature 241: 543–545 (1973).
Baker, HG, Baker, I: Studies of nectar-constitution and pollinatorplant coevolution. In: Gilbert, LE, Raven, PH (eds) Coevolution of animals and plants. Univ. of Texas Press, Austin (1975).
Baker, HG, Baker, I: Chemical constituents of nectar in relation to pollination mechanisms and phylogeny. In: Niteci, M (eds) Biochemical aspects of evolutionary biology. Univ. Chicago Press, Chicago (1981).
Bradford, MM: A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principles of protein-dye binding. Anal. Biochem. 72: 248–254 (1976).
Burquez, A, Corbet, SA: Do flowers reabsorb nectar? Funct. Ecol. 5: 369–379 (1991).
Cabras, P, Angioni, A, Tuberoso, C, Floris, I, Reniero, F, Guillou, C, Ghelli, S: Homogentisic acid: A phenolic acid as a marker of strawberry-tree (Arbutus unedo) honey. J. Agric. Food Chem. 47: 4064–4067 (1999).
Carter, C, Graham, R, Thornburg, RW: Nectarin I is a novel, soluble germin-like protein expressed in the nectar of Nicotiana sp. Plant Mol. Biol. 41: 207–216 (1999).
Carter, C, Thornburg, RW: Tobacco Nectarin I: Purification and characterization as a germin-like, manganese superoxide dismutase implicated in the defense of floral reproductive tissues. J. Biol. Chem. 275: 36726–36733 (2000).
Chomczynski, P, Sacchi, N: Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162: 156–9 (1987).
Deinzer, ML, Thompson, PA, Burgett, DM, Isaacson, DL: Pyrrolizidine alkaloids: Their occurance in honey from tansy ragwort (Senecio jacobaea L.). Science 195: 497–499 (1977).
Ecroyd, CE, Franich, RA, Kroese, HW, Steward, D: Volatile constituents of Dactylanthus taylorii flower nectar in relation to flower pollination and browsing by animals. Phytochemistry 40: 1387–1389 (1995).
Esau, K: Anatomy of seed plants. John Wiley & Sons, New York (1977).
Ferreres, F, Andrade, P, Gil, MI, Tomas Barberan, FA: Floral nectar phenolics as biochemical markers for the botanical origin of heather honey. Zeitschr. Lebensmittel Untersuch. Forsch. 202: 40–44 (1996).
Frey-Wyssling, A: The phloem supply to the nectaries. Acta Bot. Neerl. 4: 358–369 (1955).
Ge, YX, Angenent, GC, Wittich, PE, Peters, J, Franken, J, Busscher, M, Zhang, LM, Dahlhaus, E, Kater, MM, Wullems, GJ, Creemers-Molenaar, T: NEC1, a novel gene, highly expressed in nectary tissue of Petunia hybrida. Plant J 24: 725–34. (2000).
Gorman, CM, Moffat, LF, Howard, BH: Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol. Cell. Biol. 2: 1044–1051 (1982).
Griebel, C, Hess, G: The vitamin C content of flower nectar of certain Labiatae. Zeit. Untersuch. Lebensmitt. 79: 168–171 (1940).
Jackson, D, Culianez-Macia, F, Prescott, AG, Roberts, K, Martin, C: Expression patterns of myb genes from Antirrhinum flowers. Plant Cell 3: 115–125 (1991).
Kernan, A, Thornburg, RW: Auxin levels regulate the expression of a wound-inducible proteinase inhibitor II–chloramphenicol acetyl transferase gene fusion in vitro and in vivo. Plant Physiol. 91: 73–78 (1989).
Koltunow, AM, Truettner, J, Cox, KH, Walroth, M, Goldberg, RB: Different temporal and spatial gene expression patterns occur during anther development. Plant Cell 2: 1201–1224 (1990).
Park, S, Thornburg, RW: Loss of specific sequences in a natural variant of potato proteinase inhibitor II gene results in a loss of wound-inducible gene expression. Ag Chem & Biotech 39: 104–111 (1996).
Rodriguez-Arce, AL, Diaz, N: The stability of beta-carotene in mango nectar. J. Agric. Univ. P.R. Rio Piedras, P.R. 76: 101–102 (1992).
Roshchina, VV, Roshchina, VD: The excretory function of higher plants. Springer-Verlag, Berlin (1993).
Sablowski, R, Moyano, E, Culianez-Macia, F, Schuch, W, Martin, C, Bevan, M: A flower-specific Myb protein activates transcription of phenylpropanoid biosynthetic genes. EMBO J 13: 128–37 (1994).
Smith, LL, Lanza, J, Smith, GC: Amino acid concentrations in extrafloral nectar of Impatiens sultani increase after simulated herbivory. Ecol. Publ. Ecol. Soc. Am. 71: 107–115 (1990).
Thornburg, RW, An, G, Cleveland, TE, Johnson, R, Ryan, CA: Wound-inducible expression of a potato inhibitor IIchloramphenicol acetyltransferase gene fusion in transgenic tobacco plants. Proc. Natl. Acad. Sci. USA 84: 744–748 (1987).
Vogel, S: Flowers offering fatty oil instead of nectar. Abstracts XIth Internatl. Bot. Congr. Seattle (1969).
Author information
Authors and Affiliations
Corresponding author
Additional information
†Dedicated in memoriam to Dr. Jubran Wakim, Department of Chemistry, Middle Tennessee State University USA
Rights and permissions
About this article
Cite this article
Carter, C., Thornburg, R.W. The nectary-specific pattern of expression of the tobacco Nectarin I promoter is regulated by multiple promoter elements† . Plant Mol Biol 51, 451–457 (2003). https://doi.org/10.1023/A:1022370203570
Issue Date:
DOI: https://doi.org/10.1023/A:1022370203570