Abstract
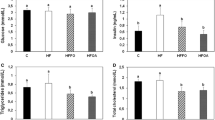
Atherosclerosis-mediated coronary artery disease is a significant cause of mortality in lupus patients. Both an activated immune system and hyperlipidemia are implicated in the pathogenesis of the atherosclerotic lesions of lupus. In this study, the increases in anticardiolipin antibodies, total cholesterol, and LDL cholesterol with age were significantly lowered by fish oil and food restriction, either alone or in combination. Food restriction also significantly decreased the elevation in anti-dsDNA antibody production seen with age in ad libitum groups. Interestingly, effects of food restriction and fish oil on both lipid profile and autoantibody production were seen from a young age. Accumulation of leukocytes in the blood vessels and deposition of IgG in the glomerular mesangium also were suppressed by food restriction. Thus, beneficial effects of fish oil and food restriction on lupus nephritis and survival could be, at least in part, due to their selective effect on atherogenic risk factors.
Similar content being viewed by others
References
Karrar A, Sequeira W, Block JA: Coronary artery disease in systemic lupus erythematosus: A review of the literature. Semin Arthritis Rheum 30:436-443, 2001
Petri M, Perez-Gutthann S, Spence D, Hochberg MC: Risk factors for coronary artery disease in patients with systemic lupus erythematosus. Am J Med 93:513-519, 1992
Andany MA, Kasiske BL: Dyslipidemia and its management after renal transplantation. J Nephrol 14 Suppl 4:S81-88, 2001
Baum CL, Thielke K, Westin E, Kogan E, Cicalese L, Benedetti E: Predictors of weight gain and cardiovascular risk in a cohort of racially diverse kidney transplant recipients. Nutrition 18:139-146, 2002
Wierzbicki AS: The role of lipid lowering in transplantation. Int J Clin Pract 53:54-59, 1999
Kobashigawa JA, Kasiske BL: Hyperlipidemia in solid organ transplantation. Transplantation 63:331-338, 1997
Arnadottir M, Berg AL: Treatment of hyperlipidemia in renal transplant recipients. Transplantation 63:339-345, 1997
Zuckerman E, Toubi E, Shiran A, Sabo E, Shmuel Z, Golan TD, Abinader E, Yeshurun D: Anticardiolipin antibodies and acute myocardial infarction in nonsystemic lupus erythmatosus patients: A controlled prospective study. Am J Med 101:381-386, 1996
Gogos CA, Skoutelis A, Kalfarentzos F: The effects of lipids on the immune response of patients with cancer. J Nutr Health Aging 4:172-175, 2000
Iso H, Rexrode KM, Stampfer MJ, Manson JE, Colditz GA, Speizer FE, Hennekens CH, Willett WC: Intake of fish and omega-3 fatty acids and risk of stroke in women. JAMA 285:304-312, 2001
James MJ, Cleland LG: Dietary n-3 fatty acids and therapy for rheumatoid arthritis. Semin Arthritis Rheum 27:85-97, 1997
Yamada T, Strong JP, Ishii T, Ueno T, Koyama M, Wagayama H, Shimizu A, Sakai T, Malcom GT, Guzman MA: Atherosclerosis and omega-3 fatty acids in the populations of a fishing village and a farming village in Japan. Atherosclerosis 153:469-481, 2000
Das UN: Beneficial effect(s) of n-3 fatty acids in cardiovascular diseases: But, why and how? Prostaglandins Leukot Essent Fatty Acids 63:351-362, 2000
Fernandes G: Effect of calorie restriction and omega-3 fatty acids on autoimmunity and aging. Nutr Rev 53:S72-S79, 1995
Troyer DA, Chandrasekar B, Thinnes T, Stone A, Loskutoff DA, Fernandes G: Effects of energy intake on type I plasminogen activator inhibitor levels in glomeruli of lupus-prone B/W mice. Am J Pathol 146:111-120, 1995
Mizutani H, Kurata Y, Kosugi S, Shiraga M, Kashiwagi H, Tomiyama Y, Kanakura Y, Good RA, Matsuzawa Y: Monoclonal anticardiolipin autoantibodies established from the (New Zealand white × BXSB)F1 mouse model of antiphospholipid syndrome cross-react with oxidized low-density lipoprotein. Arthritis Rheum 38:1382-1388, 1995
Theofilopoulos AN, Kofler R, Singer PA, Dixon FJ: Molecular genetics of murine lupus models. Adv Immunol 46:61-109, 1989
McNeil HP, Chesterman CN, Krilis SA: Immunology and clinical importance of antiphospholipid antibodies. Adv Immunol 49:193-280, 1991
Roubey RA: Autoantibodies to phospholipid-binding plasma proteins: A new view of lupus anticoagulants and other “antiphospholipid” autoantibodies. Blood 84:2854-2867, 1994
Vaarala O, Puurunen M, Lukka M, Alfthan G, Leirisalo-Repo M, Aho K, Palosuo T: Affinity-purified cardiolipin-binding antibodies show heterogeneity in their binding to oxidized low-density lipoprotein. Clin Exp Immunol 104:269-274, 1996
Vaarala O: Antiphospholipid antibodies and myocardial infarction. Lupus 7:S132-134, 1988
Fernandes G, Good RA: Inhibition by restricted-calorie diet of lymphoproliferative disease and renal damage in MRL/lpr mice. Proc Natl Acad Sci USA 81:6144-6148, 1984
Fernandes G, Bysani C, Venkatraman JT, Tomar V, Zhao W: Increased TGF-beta and decreased oncogene expression by omega-3 fatty acids in the spleen delays onset of autoimmune disease in B/W mice. J Immunol 152:5979-5987, 1994
Jolly C, Muthukumar A, Reddy Avula C, Troyer D, Fernandes G: Life span is prolonged in food-restricted autoimmune-prone (NZB × NZW)F(1) mice fed a diet enriched with (n-3) fatty acids. J Nutr 131:2753-2760, 2001
Muthukumar AR, Jolly CA, Zaman K, Fernandes G: Calorie restriction decreases proinflammatory cytokines and polymeric Ig receptor expression in the submandibular glands of autoimmune prone (NZB × NZW)F1 mice. J Clin Immunol 20:354-361, 2000
Jolly CA, Fernandes G: Diet modulates Th-1 and Th-2 cytokine production in the peripheral blood of lupus-prone mice. J Clin Immunol 19:172-178, 1999
Barnes JL, Hastings RR, De la Garza MA: Sequential expression of cellular fibronectin by platelets, macrophages, and mesangial cells in proliferative glomerulonephritis. Am J Pathol 145:585-589, 1994
Hornbeck P: Antibody detection and preparation. In Current Protocols in Immunology, Colligan J, Krusbeek A, Margulies D, Shevach E, Strober W (eds). New York, John Wiley & Sons, 1992, pp 231-234
Norris MS, McConnell TJ, Mannie MD: Interleukin-2 promotes antigenic reactivity of rested T cells but prolongs the postactivational refractory phase of activated T cells. Cell Immunol 211:51-60, 2001
Rahman P, Gladman DD, Urowitz MB, Yuen K, Hallett D, Bruce IN: The cholesterol lowering effect of antimalarial drugs is enhanced in patients with lupus taking corticosteroid drugs. J Rheumatol 26:325-330, 1999
Wallace DJ, Metzger AL, Stecher VJ, Turnbull BA, Kern PA: Cholesterol-lowering effect of hydroxychloroquine in patients with rheumatic disease: Reversal of deleterious effects of steroids on lipids. Am J Med 89:322-326, 1990
Ilowite NT, Copperman N, Leicht T, Kwong T, Jacobson MS: Effects of dietary modification and fish oil supplementation on dyslipoproteinemia in pediatric systemic lupus erythematosus. J Rheumatol 22:1347-1351, 1995
Chang SC, Chiang BL, Wu WM, Lin BF: Different dietary fats influence serum and tissue lipids and anti-cardiolipin antibody levels in autoimmune-prone NZB/W F1 mice. Br J Nutr 81:331-340, 1999
Kannel WB, Castelli WP, Gordon T, McNamara PM: Serum cholesterol, lipoproteins, and the risk of coronary heart disease. The Framingham study. Ann Intern Med 74:1-12, 1971
Manzi S, Selzer F, Sutton-Tyrrell K, Fitzgerald SG, Rairie JE, Tracy RP, Kuller LH: Prevalence and risk factors of carotid plaque in women with systemic lupus erythematosus. Arthritis Rheum 42:51-60, 1999
Leong KH, Koh ET, Feng PH, Boey ML: Lipid profiles in patients with systemic lupus erythematosus. J Rheumatol 21:1264-1267, 1994
Hearth-Holmes M, Baethge BA, Broadwell L, Wolf RE: Dietary treatment of hyperlipidemia in patients with systemic lupus erythematosus. J Rheumatol 22:450-454, 1995
Hung P, Gu JY, Kaku S, Yunoki S, Ohkura K, Ikeda I, Tachibana H, Sugano M, Yazawa K, Yamada K: Dietary effects of eicosapentaenoic and docosahexaenoic acid esters on lipid metabolism and immune parameters in Sprague-Dawley rats. Biosci Biotechnol Biochem 64:2588-2593, 2000
Harris WS: Fish oil and plasma lipid and lipoprotein metabolism in humans: A critial review. J Lipid Res 30:785-807, 1989
Clark WF, Parbtani A, Naylor CD, Levinton CM, Muirhead N, Spanner E, Huff MW, Philbrick DJ, Holub BJ: Fish oil in lupus nephritis: Clinical findings and methodological implications. Kidney Int 44:75-86, 1993
Leon TI, Lim BO, Yu BP, Lim Y, Jeon EJ, Park DK: Effect of dietary restriction on age-related increase of liver susceptibility to peroxidation in rats. Lipids 36:589-593, 2001
Verdery RB, Ingram DK, Roth GS, Lane MA: Caloric restriction increases HDL2 levels in rhesus monkeys (Macaca mulatta). Am J Physiol 273:E714-719, 1997
Choi YS, Goto S, Ikeda I, Sugano M: Age-related changes in lipid metabolism in rats: The consequence of moderate food restriction. Biochim Biophys Acta 963:237, 1998
Hubert MF, Laroque P, Gillet JP, Keenan KP: The effects of diet, ad libitum feeding, and moderate and severe dietary restriction on body weight, survival, clinical pathology parameters, and cause of death in control Sprague-Dawley rats. Toxicol Sci 58:195-207, 2000
Ross R: The pathogenesis of atherosclerosis: A perspective for the 1990s. Nature 362:801-809, 1993
Steinberg D, Parthasarathy S, Crew TE, Khoo JC, Witztum JL: Beyond cholesterol: Modification of low density lipoproteins that increases its atherogenicity. N Engl J Med 320:915-924, 1989
Libby P, Hansson GK: Involvement of the immune system in human atherogenesis: Current knowledge and unanswered questions. Lab Invest 64:5-15, 1991
Wick G, Schett G, Amberger A, Kleindienst R, Xu Q: Is atherosclerosis an immunologically mediated disease? Immunol Today 16:27-33, 1995
Kishikawa H, Shimokama T, Watanabe T: Localization of T lymphocytes and macrophages expressing IL-1, IL-2 receptor, IL-6 and TNF in human aortic intima. Role of cell-mediated immunity in human atherogenesis. Virchows Arch A Pathol Anat Histopathol 423:433-442, 1993
van der Wal AC, Das PK, Bentz van de Berg D, van der Loos CM, Becker AE: Atherosclerotic lesions in humans. In situ immunophenotypic analysis suggesting an immune mediated response. Lab Invest 61:166-170, 1989
Adams DH, Shaw S: Leucocyte—endothelial interactions and regulation of leucocyte migration [see comments]. Lancet 343:831-836, 1994
Kabakov A, Tertov V, Saenko V, Poverenny A, Orekhov A: The atherogenic effect of lupus sera: Systemic lupus erythematosus-derived immune complexes stimulate the accumulation of cholesterol in cultured smooth muscle cells from human aorta. Clin Immunol 63:214-220, 1992
George J, Blank M, Levy Y, Meroni P, Damianovich M, Tincani A, Shoenfeld Y: Differential effects of anti-beta2-glycoprotein I antibodies on endothelial cells and on the manifestations of experimental antiphospholipid syndrome. Circulation 97:900-906, 1998
Ameli S, Hultgardh-Nilsson A, Regnstrom J, Calara F, Yano J, Cercek B, Shah PK, Nilsson J: Effect of immunization with homologous LDL and oxidized LDL on early atherosclerosis in hypercholesterolemic rabbits. Arterioscler Thromb Vasc Biol 16:1074-1079, 1996
Witztum JL: The oxidation hypothesis of atherosclerosis. Lancet 344:793-795, 1994
Salonen JT, Yla-Herttuala S, Yamamoto R, Butler S, Korpela H, Salonen R, Nyyssonen K, Palinski W, Witztum JL: Autoantibody against oxidised LDL and progression of carotid atherosclerosis. Lancet 339:883-887, 1992
Palinski W, Yla-Herttuala S, Rosenfeld ME, Butler SW, Socher SA, Parthasarathy S, Curtiss LK, Witztum JL: Antisera and monoclonal antibodies specific for epitopes generated during oxidative modification of low density lipoprotein. Arteriosclerosis 10:325-335, 1990
Matsuura E, Kobayashi K, Yasuda T, Koike T: Antiphospholipid antibodies and atherosclerosis. Lupus 7:S135-139, 1998
George J, Blank M, Hojnik M, Bar-Meir E, Koike T, Matsuura E, Lorber M, Aviram M, Shoenfeld Y: Oxidized low-density lipoprotein (Ox-LDL) but not LDL aggravates the manifestations of experimental antiphospholipid syndrome (APS). Clin Exp Immunol 108:227-233, 1997
Vaarala O, Alfthan G, Jauhiainen M, Leirisalo-Repo M, Aho K, Palosuo T: Cross-reaction between antibodies to oxidised low-density lipoprotein and to cardiolipin in systemic lupus erythematosus. Lancet 341:923-925, 1993
Shevach EM, McHugh RS, Piccirillo CA, Thornton AM: Control of T-cell activation by CD4+ CD25+ suppressor T cells. Immunol Rev 182:58-67, 2001
Sakaguchi S, Sakaguchi N, Shimizu J, Yamazaki S, Sakihama T, Itoh M, Kuniyasu Y, Nomura T, Toda M, Takahashi T: Immunologic tolerance maintained by CD25+ CD4+ regulatory T cells: Their common role in controlling autoimmunity, tumor immunity, and transplantation tolerance. Immunol Rev 182:18-32, 2001
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Muthukumar, A., Zaman, K., Lawrence, R. et al. Food Restriction and Fish Oil Suppress Atherogenic Risk Factors in Lupus-Prone (NZB × NZW) F1 Mice. J Clin Immunol 23, 23–33 (2003). https://doi.org/10.1023/A:1021996130672
Issue Date:
DOI: https://doi.org/10.1023/A:1021996130672