Abstract
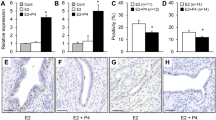
Tamoxifen has been widely used for treatment, and more recently, for the prevention of breast cancer. Since breast carcinomas are composed of heterogeneous populations of estrogen receptor-positive (ER+) cells, we hypothesized that tamoxifen may suppress tumor growth by differentially affecting cell proliferation and apoptosis. ER+ mammary tumors were induced in Sprague–Dawley rats by N-methyl-N-nitrosourea (MNU) and when they became palpable, the animals were treated for 5, 10, or 20 days with tamoxifen, 1.0 mg/kg body weight. Tamoxifen induced a time-dependent decrease in proliferating (BrdU-labeled) cells, arrested the cells in G1/0 phase, and differentially decreased the cyclin E and cyclin D1 expression at mRNA and protein levels. In the same tumors, apoptotic cells increased during the first 10 days of treatment, but their number remained unchanged with extension of the treatment to 20 days. Thus, we provide data that tamoxifen may differentially affect cell proliferation and apoptosis in mammary tumors and that the expression levels of cyclin D1 and cyclin E might also be considered potential intermediate biomarkers of response of mammary tumors to tamoxifen and possibly to other selective estrogen receptor modulators (SERMs).
Similar content being viewed by others
References
Osborne CK: Tamoxifen in the treatment of breast cancer. N Engl J Med 339: 1609-1618, 1998
Fisher B, Digman J, Wolmark N, Wickerham DL, Fisher ER, Mamounas E, Smith R, Begovic M, Dimitrov NV, Margolese RJ, Kardinal CG, Kavanah MT, Fahrenbacher L, Oishi RH: Tamoxifen in treatment of intraductal breast cancer. National Surgical Ajuvant Breast and Bowel Project B-24 randomized control trial. Lancet 353: 1993-2000, 1999
Fisher B, Costantino JP, Wicckerham DL, Redmond CK, Kavanah M, Cronin WM, Vogel V, Robidox A, Dimitrov N, Atkins J, Daley M, Wieand S, Tan-Chiu E, Ford L, Wolmark N: Tamoxifen for prevention of breast cancer. Report of the National Surgical Adjuvant Breast and Bowel Project, P-1 study. J Natl Cancer Inst 90: 1371-1388, 1998
Jordan VG, Morrow M: Tamoxifen, reloxifene, and the prevention of breast cancer. Endocr Rev 20(3): 253-278, 1999
Shiau AK, Barstad D, Radek JT, Meyers MJ, Nettles KW, Katzenellenbogen BS, Katzenellenbogen JA, Agard DA, Greene GL: Structural characterization of a subtype-selective ligand reveals a novel mode of estrogen receptor antagonism. Nat Struct Biol 9(5): 359-364, 2002
Brzozowski AM, Pike ACW, Dauter Z, Hubbard RE, Bond T, Engstrom O, Ohman L, Greene GL, Gustafson JA, Carlquist M: Molecular bases of agonism and antagonism in the estrogen receptor. Nature 389: 753-758, 1997
Grese TA, Sluka JP, Bryant HU, Cullinan GJ, Glasebrook AL, Jones CD, Matsumoto K, Palkowitz AD, Sato M, Termine JD, Winter MA, Yang NN, Dodge JA: Molecular determinants of tissue selectivity in estrogen receptor modulators. Proc Natl Acad Sci USA 94(25): 14105-14110, 1997
Huggins C, Grand LC, Brillantes FP: Mammary cancer induced by a single feeding of polynuclear hydrocarbons and its suppression. Nature (Lond) 189: 204-297, 1971
Gulliano PM, Pettigrew HM, Grantham FH: Nnitrosomethylurea as a mammary gland carcinogen in rat. J Natl Cancer Inst 54: 401-414, 1975
Russo J, Gusterson BA, Rogers AE, Russo IH, Wellings SR, Zwieten MJ: Comparative study on human and rat mammary tumorigenesis. Lab Invest 62: 244-278, 1990
Jordan VC: Effect of tamoxifen (ICI 46,474) on initiation and growth of DMBA-induced rat mammary carcinoma. Eur J Cancer 12: 419-424, 1976
Gottardis MM, Jordan VC: Antitumor action of deoxyfene and tamoxifen in the N-nitroso-N-nitrosourea induced rat mammary carcinoma model. Cancer Res 47: 4020-4024, 1987
Osborn MP, Ruperto JF, Crowe JP, Rosen PP, Telang NT: Effect of tamoxifen on preneoplastic cell proliferation in N-nitroso-N-methylurea-induced mammary carcinogenesis. Cancer Res 52: 1477-1480, 1992
Johnston SRD, Boedinghouse IM, Riddler S, Haynes BP, Hardcastle IR, Rowland M, Grimshaw R, Jarman M, Dawsett M: Idoxifene antagonizes estradiol-dependant MCF-7 breast cancer xenografts growth through sustain induction of apoptosis. Cancer Res 59: 3646-3651, 1999
Huovinen R, Warri A, Collan Y: Mitotic activity, apoptosis, and TRPM-2 mRNA expression in DMBA-induced rat mammary carcinoma treated with anti-estrogen toremifene. Int J Cancer 55: 685-691, 1993
Christov K, Shilkaitis A, Green A, Mehta RG, Grubbs C, Kelloff G, Lubet R: Cellular responses of mammary tumors to aromatase inhibitors: effects of vorozole. Breast Cancer Res Treat 60(2): 117-128, 2000
Shilkaitis A, Green A, Graves J, Mehta RR, Lubet R, Steele V, Kelloff G, Christov K: Bcl-2 and Bax are differentially expressed in hyperplastic, premalignant, and malignant lesions of mammary carcinogenesis. Cell Growth Differ 11: 437-445, 2000
Mandlekar S, Hebber V, Christov K, Kong TAN: Pharmacodynamics of tamoxifen and its 4-hydroxy and N-desmathyl metabolites: activation of caspases and induction of apoptosis in rat mammary tumors and in human breast cancer cell lines. Cancer Res, 2000
Sgambato A, Han EK, Zhang YJ, Moon RC, Santella RM, Weinstein IB: Deregulated expression of cyclin D1 and other cell cycle related genes in carcinogen-induced rat mammary tumors. Carcinogenesis 16: 2193-2198, 1995
Christov K, Ikui A, Shilkaitis A, Green A, Najmabadi F, Lubet R, Kelloff G, Weinstein IB: Tamoxifen selectively modulated the cell cycle regulatory proteins in rat mammary tumors. Proc 92nd AACR Meeting 42: 911, 2001
Shilkaitis A, Green A, Lubet R, Steele V, Kelloff G, Christov K: Neoplastic transformation of mammary epithelial cells in rats is associated with decreased apoptotic cell death. Carcinogenesis 21: 227-233, 2000
Gavrielli Y, Sherman Y, Ben-Sasson SA: Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol 119: 493-501, 1992
Christov K, Milev A, Todorov C: DNA aneuploidy and cell proliferation in breast carcinomas. Cancer 64: 673-679, 1989.
Vindelov LL, Christiancen IJ: A review of techniques and results obtained in one laboratory by an integrated system of methods designed for routine clinical flow cytometry DNA analysis. Cytometry 11: 753-770, 1990.
Snedecor GJW, Cochran WG: Statistical Methods. 8th edn, Iowa State University Press, Ames, IA, Chapter 11, 1968.
Jang TJ, Park JH, Cho MY, Kim JR: Chemically induced rat mammary tumors treated with tamoxifen showed decreased expression of cyclin D1, cyclin E, and p21(Cip1). Cancer Lett 170(2): 109-116, 2001
Lubet R, You M, Ruchon D, End W, Wouters W, Christov K, Grubbs C: Therapeutic and chemopreventive effects of R115777, a farnesyl transferase inhibitor (FTI) on N-methyl-N-nitrosourea (MNU) induced rat mammary cancer and effects on potential biomarkers. Proc AACR-NCI-EORTC 7: 3745, 2001
Musgrove EA, Hui R, Sweeney KJE, Watts CKW, Sutherland RL: Cyclins and breast cancer. J Mamm Gland Biol Neoplasia 1(2): 153-164, 1996
Lundberg ASD, Weinberg RA: Control of the cell cycle. Eur J Cancer 35: 1886-1894, 1999
Weinstein BI, Begemann M, Zhou P, Han EKH, Sgambato A, Doki Y, Arber N, Ciaparrone M, Yamamoto H: Disorders in cell circuity associated with multistage carcinogenesis: Exploitable targets for cancer prevention and therapy. Clinical Cancer Res 3: 2696-2702, 1997.
Ip C, Thompson HJ, Ganther HE: Selenium modulation of cell proliferation and cell cycle biomarkers in normal and premalignant cells of the rat mammary gland. Cancer Epidem Biomarkers Prevent 9: 49-54, 2000
Ip C, Dong Y: Methylselenocysteine modulates proliferation and apoptosis biomarkers in premalignant lesions of the rat mammary gland. Anticancer Res 21(2A): 863-867, 2001
Zhu Z, Jiang W, Thompson HJ: Effects of energy restriction on the expression of cyclin D1 and p27 during premalignant and malignant stages of chemically induced mammary carcinogenesis. Mol Carcin 24: 241-245, 1999
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Christov, K., Ikui, A., Shilkaitis, A. et al. Cell Proliferation, Apoptosis, and Expression of Cyclin D1 and Cyclin E as Potential Biomarkers in Tamoxifen-Treated Mammary Tumors. Breast Cancer Res Treat 77, 253–264 (2003). https://doi.org/10.1023/A:1021804121171
Issue Date:
DOI: https://doi.org/10.1023/A:1021804121171