Abstract
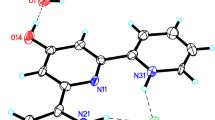
Crystal structure determinations of the three title hexahalogenated bipyrroles, (I) C10H6Br6N2, (II) C10H6Br4Cl2N2, and (III) C10H6Cl6N2, reveal essentially planar pyrrole rings having dihedral angles of 64.7, 65.1, and 64.2° between the least-squares planes, favoring in each case a closer methyl–halogen conformation. All three complexes crystallize in the orthorhombic space group Pbcn with the following cell dimensions: (I) a = 12.654(3) Å, b = 8.853(2) Å, c = 13.753(3) Å, α = β = γ = 90°, Z = 4; (II) a = 12.438(6) Å, b = 8.753(6) Å, c = 13.696(3) Å, α = β = γ = 90°, Z = 4; (III) a = 12.088(6) Å, b = 8.566(4) Å, c = 13.486(8) Å, α = β = γ = 90°, Z = 4.
Similar content being viewed by others
References
Tittlemier, S.A.; Simon, M., Jarman, W.M.; Elliott, J.E.; Norstrom, R.J. Environ. Sci. Technol. 1999, 33, 26–33.
Gribble, G.W.; Blank, D.H.; Jasinski, J.P. Chem. Commun. 1999, 2195–2196.
Gribble, G.W. Prog. Chem. Org. Nat. Prod. 1996, 68, 1–498.
Gribble, G.W. Chem. Soc. Rev. 1999, 28, 335–346.
Gribble, G.W. Environ. Sci. Pollut. Res. 2000, 7, 37–49.
Sheldrick, G.M. SHELX-97, Program for Crystal Structure Solution and Refinement; University ofGöttingen: Germany, 1997.
Berskens, P.T.; Admiral, G.; Berskens, G.; Bosman, W.P.; de Gelder, R.; Israel, R.; Smits, J.M.M.; The DIRDIF-94 Program System; Technical Report of the Crystallography Laboratory: University of Nijmegen: The Netherlands, 1994.
Cromer, D.T.; Waber, J.T. International Tables for X-ray Crystallography; Kynoch Press: Birmingham, England, 1974; Vol. IV; Table 2.2A.
Ibers, J.A.; Hamilton, W.C. Acta Crystallogr. 1964, 17, 781.
Creagh, D.C.; McAuley, W.J. International Tables for Crystallography (Wilson, A.J.C., Ed.); Kluwer Academic Publishes: Boston, 1992; Vol C, pp. 219–222; Table 4.2.6.8.
Creagh, D.C.; Hubbell, J.H. International Tables for Crystallography (Wilson, A.J.C., Ed.); Kluwer Academic Publishes: Boston, 1992; Vol. C, pp. 200–206; Table 4.2.4.3.
TeXsan for Windows, version 1.06: Crystal Structure Analysis Package; Molecular Structure Corp. The Woodlands, Texas. 1997–1999.
Gordon, A.J.; Ford, R.A. The Chemists Companion, A Handbook of Practical Data, Techniques, and References; Wiley-Interscience, New York, 1972; p. 108.
Brown, G.M.; Strydom, O.A.W. Acta Crystallogr., Sect. B. 1974, 30, 801–804.
Pedersen, B.F. Acta Crystallogr., Sect. B. 1975, 31, 2931–2933.
Singh, P.; McKinney, J.D. Acta Crystallogr., Sect. B. 1979, 35, 259–262.
Field, L.D.; Skelton, B.W.; Sternhell, S.; White, A.H. Aust. J. Chem. 1985, 38, 391–399.
Fedoreyev, S.A.; Ilyin, S.G.; Utkina, N.K.; Maximov, O.B.; Reshetnyak, M.V.; Antipin, M.Yu.; Struchkov, Yu.T. Tetrahedron 1989, 45, 3487–3492.
Vilkov, L.V.; Akishin, P.A.; Presnyakova, V.M. Zh. Strukt. Khim. 1962, 3, 5–9.
Merz, A.; Kronberger, J.; Dunsch, L.; Neudeck, A.; Petr, A.; Parkanyi, L. Angew. Chem. Int. Ed. 1999, 38, 1442–1446.
Rømming, C.H.R.; Seip, H.M.; Aanesen Øymo, I.-M. Acta Chem. Scand. Ser. A 1974, 28, 507–514.
Dewar, M.J.S.; Schmeising, H.N. Tetrahedron 1960, 11, 96–120.
Trotter, J. Acta Crystallogr 1961, 14, 1135–1140.
Bastiansen, O.; Trætteberg, M. Tetrahedron 1962, 17, 147–154.
Nogami, T.; Shigihara, Y.; Matsuda, N.; Takahashi, Y.; Naganawa, H.; Nakamura, H.; Hamada, M.; Muraoka, Y.; Takita, T.; Iitaka, Y.; Takeuchi, T. J. Antibiot. 1990, 43, 1192–1194.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Blank, D.H., Gribble, G.W., Schneekloth, J.S. et al. A novel class of naturally occurring halogenated pyrroles, 1,1′-dimethyl-3,3′,4,4′,5,5′-hexabromo-2,2′-bipyrrole, 5,5′-dichloro-1,1′-dimethyl-3,3′,4,4′-tetrabromo-2,2′-bipyrrole, and 1,1′-dimethyl-3,3′,4,4′,5,5′-hexachloro-2,2′-bipyrrole. Journal of Chemical Crystallography 32, 541–546 (2002). https://doi.org/10.1023/A:1021117431367
Issue Date:
DOI: https://doi.org/10.1023/A:1021117431367