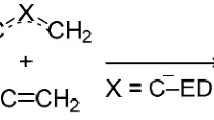Abstract
The structure of [3.3.2]- and [3.3.3]propellanes, their framework analogs, and their radical-cations was investigated by computational methods (BLYP and B3LYP) in the 6-31G* basis set. The reactivity of the propellanes toward model oxidizing electrophiles does not contradict the quantum-chemical calculations. In the case of the tetracyclic framework analog of [3.3.3]propellane the reaction takes place as C—H substitution, whereas in the case of [3.3.2]propellanes it takes place as C—C-oxidative addition.
Similar content being viewed by others
REFERENCES
K. B. Wiberg, Chem. Rev., 89, 975–983 (1989).
A. A. Fokin, P. A. Gunchenko, S. A. Peleshanko, et al., Eur. J. Org. Chem., 855–860 (1999).
G. A. Olah, R. Penner, P. Schilling, and K. M. Yoke, J. Am. Chem. Soc., 95, 7686–7692 (1973).
A. A. Fokin, P. A. Gunchenko, N. I. Kulik, et al., Tetrahedron, 52, 5857–5866 (1996).
A. A. Fokin, P. A. Gunchenko, and B. A. Tkachenko, Tetrahedron Lett., 38, 639–642 (1997).
A. A. Fokin, P. R. Schreiner, P. A. Gunchenko, et al., J. Am. Chem. Soc., 122, 7317–7326 (2000).
A. A. Fokin, S. A. Peleshanko, P. A. Gunchenko, et al., Eur. J. Org. Chem., 3357–3362 (2000).
A. A. Fokin, P. R. Schreiner, P. v. R. Schleyer, et al., J. Org. Chem., 19, 6494–6502 (1998).
S. G. Lias, J. E. Bartmess, J. F. Liebman, et al., J. Phys. Chem. Ref. Data, 17, 1 (1988).
L. Eberson and F. Radner, Accounts Chem. Res., 20, 53–59 (1987).
G. A. Olah, P. Ramaiah, C. B. Rao, et al., J. Am. Chem. Soc., 115, 7246–7249 (1993).
I. K. Moiseev, Yu. N. Klimochkin, M. N. Zemtsova, et al., Zh. Org. Khim., 20, 1435–1438 (1984).
M. J. Frisch, G. W. Trucks, H. B. Schlegel, et al., Gaussian 98, Revision A.7, Gaussian, Inc., Pittsburgh, PA (1998).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Shubina, T.E., Gunchenko, P.A., Vigovskaya, T.S. et al. [3.3.2]- and [3.3.3]Propellanes in Reactions with Oxidizing Electrophiles. Theoretical and Experimental Chemistry 38, 229–236 (2002). https://doi.org/10.1023/A:1020511730749
Issue Date:
DOI: https://doi.org/10.1023/A:1020511730749