Abstract
Purpose. To develop a non-invasive animal model suitable for studies of altered gastroduodenal (GD) permeability, which is suggested to indicate GD damage; to validate a low cost and convenient assay for sucrose in urine, a permeability marker of GD.
Methods. Control (n = 87) and treated male Sprague-Dawley rats were dosed orally with 1 g of sucrose. Urinary excretion of the sucrose (0–8 h) was measured indirectly by cleavage to glucose and subsequent measurement of glucose in urine using a calorimetric assay. Treated rats were administered single oral doses of 10 and 20 mg/kg indomethacin, or 42 mg/kg aspirin alone or with 0.5 mL 50% ethanol (n = 7 in each group).
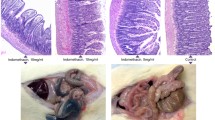
Results. The assay was linear within the examined range of 10–100 ug/mL sucrose. The inter and intraday variations were 7.63% and 6.89%, respectively. The urinary excretion of sucrose was complete in 8 h. In control rats the urinary excretion of sucrose exhibited a left skewed frequency distribution curve with a mean of 0.6 ± 0.14% of the dose excreted. All treatment, with the exception of 10 mg/kg indomethacin significantly increased the GD permeability. The GD effect was found to be dose dependent and parallels those reported for humans.
Conclusions. The rat is a suitable model for studies of GD permeability. Combined use of sucrose and 51Cr-EDTA, a marker of intestinal permeability, allows for non-invasive examination of abnormalities of the entire gut. The sucrose assay is convenient and cost effective. The rat model may be useful in the preclinical screening of NSAID formulations and also in the detection of other GI abnormalities.
Similar content being viewed by others
REFERENCES
J. F. Fries. NSAID gastropathy: the second most deadly rheumatic disease? Epidemiology and risk appraisal. J. Rheumatology (Supp 28) 18:7–11 (1991).
K. D. Rainsford. Handbook of Animal Models for Rheumatic Diseases, RA Greenwald, R. A.; Diamond, H. S., Eds.; CRC Press: Boca Raton, pp. 181–206, 1988.
D. W. Kaufman, J. P. Kelly, J. E. Sheehan, A. Laszlo, B. E. Wilholm, L. Alfredsson, R. S. Koff, and S. Shapiro. Nonsteroidal anti-inflammatory drug use in relation to major upper gastrointestinal bleeding. Clin. Pharm. Ther. 53:485–494 (1993).
L. Aabakken, S. Larsen, and M. Osnes. Visual analogue scales for endoscopic evaluation of nonsteroidal anti-inflammatory drug-induced mucosal damage in the stomach and duodenum. Scand. J. Gastroenterol. 25:443–448 (1990).
J. B. Meddings, L. R. Sutherland, N. I. Byles, and J. L. Wallace. Sucrose: A Novel Permeability Marker for Gastroduodenal Disease. Gastroenterology 104:1619–1626 (1993).
L. R. Sutherland, M. Verhoef, J. L. Wallace, G. Van Rosendaal, R. Crutcher, and J. B. Meddings. A Simple, Non-invasive Marker of Gastric Damage: Sucrose Permeability. Lancet 343:998–1000 (1994).
I. Bjarnason, P. Williams, P. Smethurst, T. J. Peters, and A. J. Levi. Effect of non-steroidal anti-inflammatory drugs and prostaglandins on the permeability of the human small intestine. Gut 27:1292–1297 (1986).
J. L. Wallace, and D. N. Granger. (1992) Pathogenesis of NSAID gastropathy: are neutrophils the culprits? TiPs 13:129–131 (1992).
N. M. Davies, M. R. Wright, and F. Jamali. Antiinflammatory Drug-Induced Intestinal Permeability: The Rat a Suitable Model. Pharm. Res. 11:1564–1569 (1994)
M. S. Ali. Simultaneous determination of dextrose, sucrose, maltose, and lactose in sausage products by liquid chromatography. J. Assoc. Off. Anal. Chem. 71(6):1097–1100 (1988).
I. Bjarnason, G. Zanelli, T. Smith, P. Prouse, P. Williams, P. Smethurst, G. Delacey, M. J. Gumpel, and A. J. Levi. Nonsteroidal antiinflammatory drug-induced intestinal inflammation in humans. Gastroenterology 93:480–489 (1987)
I. Bjarnason, P. Williams, A. So, G. Zanelli, A. J. Levi, M. J. Gumple, T. J. Peters, and B. Ansell. Intestinal permeability and inflammation in rheumatoid arthritis: Effects of non-steroidal antiinflammatory drugs. Lancet 2:1171–4 (1984).
M. Rowland, and S. Reigelman, Pharmacokinetics of acetylsalicylic acid and salicylic acid after iv administration in man. J. Pharm. Sci. 2:1313–1319 (1968).
A. A. Rabassa, R. Goodgame, F. M. Sutton, C. N. Ou, C. Rognerud, and D. Y. Graham. Effect of H. Pylori infection on gastroduodenal mucosal permeability. Am. J. Gastr. 89(8):A181 1330 (1994).
G. Vettorazzi and I. MacDonald, Sucrose, Nutritional and Safety Aspects, Springer-Verlag, New York, 35–38 (1988)
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Davies, N.M., Corrigan, B.W. & Jamali, F. Sucrose Urinary Excretion in the Rat Measured Using a Simple Assay: A Model of Gastroduodenal Permeability. Pharm Res 12, 1733–1736 (1995). https://doi.org/10.1023/A:1016221923679
Issue Date:
DOI: https://doi.org/10.1023/A:1016221923679