Abstract
Purpose. Simple uncoated compressed tablets with a central hole (donut-shape) are proposed to provide a constant drug release over a long period of time (>20 hrs). The effect of hole size and drug solubility on the release kinetics is investigated.
Methods. The donut-shaped polyethylene oxide (PEO, Mw = 4 × l06) tablets (600 mg and 12 mm diameter) are bored with a drill bit (3/32″, 7/64″, 1/8″, and 5/32″).
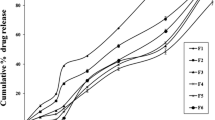
Results. The release of theophylline from the donut-shaped tablets is zero order (80 – 90% release) before rapidly decreasing. As the hole size is increased from 7/64″ to 5/32″, the release rate increases and the release time is shortened. However, the release of theophylline from the donut-shaped tablet with a hole size of 3/32″ follows the same anomalous release profile from a tablet without a hole. As drug solubility increases, the duration of linear drug release is shortened to 65 – 70% release followed by a severe tailing at the later stage of the release.
Conclusions. Donut-shaped PEO tablets with a hole provide zero-order release kinetics because the effect of the releasing surface area on the release kinetics is reduced.
Similar content being viewed by others
REFERENCES
T. Higuchi. Mechanism of sustained-action medication: theoretical analysis of rate of release of solid drugs dispersed in solid matrices. J. Pharm. Sci. 52: 1145–1149 (1963).
T. J. Roseman. Release of steroids from a silicone polymer. J. Pharm. Sci. 61: 46–50 (1972).
A. T. Pham and P. I. Lee. Proceed. Intern. Symp. Control. Rel. Bioact. Mater. 20: 220–221 (1993).
R. Bettini, P. Santi, M. T. Peracchia, P. L. Catellani, and P. Colombo. relationship between swelling and drug release in hydrogel matrix. Proceed. Intern. Symp. Control. Rel. Bioact. Mater. 20: 218–219 (1993).
R. S. Harland, A. Gazzaniga, M. E. Sangalli, P. Colombo, and N. A. Peppas. Drug/polymer matrix swelling and dissolution. Pharm. Res. 5: 488–494 (1988).
P. I. Lee and N. A. Peppas. Prediction of polymer dissolution in swellable controlled-release systems. J. Control. Release 6: 207–215 (1987).
P. Colombo, A. Gazzaniga, C. Caramelle, U. Conte, and A. La Manna. In vitro programmable zero-order release drug delivery system. Act. Pham. Technol. 33: 15–20 (1987).
G. Källstrand and B. Ekman. Membrane-coated tablets: a system for the controlled release of drugs. J. Pharm. Sc. 72: 772–775 (1983).
F. Theeuwes. Elementary osmotic pump. J. Pharm. Sci. 64: 1987–1989 (1975).
U. Conte, L. Maggi, and A. La Manna. Swellable/erodible barriers for the modulation of drug release from hydrophilic matrices. Proceed. Intern. Symp. Control. Rel. Bioact. Mater. 21: 60–61 (1994).
U. Conte, L. Maggi, P. Colombo, and A. La Manna. Multilayered hydrophilic matrices as constant release devices (Geomatrix® systems). J. Control. Release 26: 39–47 (1993).
L. Maggi, M. L. Torre, P. Giunchedi, U. Conte, and A. La Manna. New oral systems for timing-release of drugs. Proceed. Intern. Symp. Control. Rel. Bioact. Mater. 20: 366–367 (1993).
R. A. Lipper and W. Higuchi. Analysis of theoretical behavior of a proposed zero-order drug delivery system. J. Pharm. Sci. 66: 163–164 (1977).
D. S. Hsieh, W. D. Rhine, and R. Langer. Zero-order controlled release polymer matrices for micro-and macromolecules. J. Pharm. Sci. 72: 17–22 (1983).
W-Y. Kuu and H. Yalkowsky. Multiple-hole approach to zero-order release. J. Pharm. Sci. 74: 926–933 (1985).
K. G. Nelson, S. J. Smith, and R. M. Bennet. Constant-release diffusion systems: rate control by means of geometric configuration. in P. I. Lee and W. R. Good (Eds.) Controlled-Release Technology: Pharmaceutical Applications. ACS Symp. Ser. No. 348, 1987, pp324–340.
J. P. Cleave. Some geometrical considerations concerning the design of tablets. J. Pharm. Pharmacol. 17: 698–702 (1965).
A. G. Hansson, A. Giardino, J. R. Cardinal, and W. Curatolo. Perforated coated tablets for controlled release of drugs at a constant rate. J. Pharm. Sci. 77: 322–324 (1988).
M. E. Sangalli, U. Conte, A. Gazzaniga, and A. La Manna. Inert monolithic device with central hole for constant drug release. Proceed. Intern. Symp. Control. Rel. Bioact. Mater. 20: 316–317 (1993).
C. J. Kim. U.S. Patent (pending).
C. J. Kim. Drug release from compressed hydrophilic POLYOX®-WSR tablets. J. Pharm. Sci. (submitted).
A. Apicella, B. Cappello, M. A. Del Nobile, M. I. La Rotonda, G. Mensitieri, and L. Nicolais. Poly(ethylene oxide) (PEO) and different molecular weight PEO blends monolithic devices for drug release. Biomaterials 14: 83–90 (1993).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kim, Cj. Compressed Donut-Shaped Tablets with Zero-Order Release Kinetics. Pharm Res 12, 1045–1048 (1995). https://doi.org/10.1023/A:1016218716951
Issue Date:
DOI: https://doi.org/10.1023/A:1016218716951