Abstract
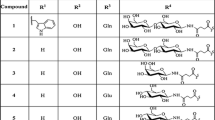
Bioreversible derivatization of TRH (pGlu–His–Pro–NH2) to protect the tripeptide against rapid enzymatic inactivation in the systemic circulation and to improve the lipophilicity of this highly hydrophilic peptide was performed by N-acylation of the imidazole group of the histidine residue with various chloroformates. Whereas TRH was rapidly hydrolyzed at its pGlu–His bond in human plasma by a TRH-specific pyroglutamyl aminopeptidase serum enzyme, the N-alkoxycarbonyl derivatives were resistant to cleavage by the enzyme. On the other hand, these derivatives are readily bioreversible as the parent TRH is formed quantitatively from the derivatives by spontaneous hydrolysis or by plasma esterase-catalyzed hydrolysis. In addition to protecting the parent TRH against rapid inactivation in the circulation and hence potentially prolonging the duration of action of TRH in vivo, the N-alkoxycarbonyl prodrug derivatives were much more lipophilic than TRH as assessed by octanol–buffer partitioning. This property may enhance prodrug penetration of the blood–brain barrier and various other biomembranes compared to the parent peptide.
Similar content being viewed by others
REFERENCES
G. Metcalf. Brain Res. Dev. 4:389–408 (1982).
I. M. D. Jackson. N. Engl. J. Med. 306:145–155 (1982).
E. C. Griffiths. Psychoneuroendocrinology 10:225–235 (1985).
E. C. Griffiths. Nature 322:212–213 (1986).
E. C. Griffiths. Clin. Sci. 73:449–457 (1987).
A. Horita, M. A. Carino, and H. Lai. Annu. Rev. Pharmacol. Toxicol. 26:311–332 (1986).
P. T. Loosen. Progr. Neuro-Psychopharmacol. Biol. Psychiat. 12:S87–S117 (1988).
G. Metcalf and I. M. D. Jackson (eds.). Ann. N.Y. Acad. Sci. 553:1–631 (1989).
M. Hichens. Drug Metab. Rev. 14:77–98 (1983).
R. M. Bassiri and R. D. Utiger. J. Clin. Invest. 52:1616–1619 (1973).
J. E. Morley, T. J. Garvin, A. E. Pekary, R. D. Utiger, M. G. Nair, C. M. Baugh, and J. M. Hershman. J. Clin. Endocrinol. Metab. 48:377–380 (1979).
L. Duntas, F. S. Keck, and E. F. Pfeiffer. Dtsch. Med. Wschr. 113:1354–1357 (1988).
E. Iversen. J. Endocrinol. 118:511–516 (1988).
C. H. Emerson. Methods Enzymol. 168:365–371 (1989).
K. Bauer. Biochimie 70:69–74 (1988).
K. Bauer and P. Nowak. Eur. J. Biochem. 99:239–245 (1979).
W. L. Taylor and J. E. Dixon. J. Biol. Chem. 253:6934–6940 (1978).
J. Møss and H. Bundgaard. Pharm. Res. 7:751–755 (1990).
W. A. Banks and A. J. Kastin. Brain Res. Bull. 15:287–292 (1985).
Y. Nagai, S. Yokohama, and Y. Nagawa. J. Pharm. Dyn. 3:500–506 (1980).
H. Bundgaard. In S. S. Davis, L. Illum, and E. Tomlinson (eds.), Delivery Systems for Peptide Drugs, Plenum Press, New York, 1986, pp. 49–68.
H. Bundgaard and J. Møss. Biochem. Soc. Trans. 17:947–949 (1989).
H. Bundgaard and J. Møss. J. Pharm. Sci. 78:122–126 (1989).
F. C. Grønvald, N. L. Johansen, and B. F. Lundt. In E. Gross and F. Meinhofer (eds.), Peptides, Structure and Biological Function, Pro. 6th Am. Peptide Symp., 1979, pp. 309–312.
F. C. Grønvald, B. F. Lundt, and N. L. Johansen. In K. Brunfeldt (ed.), Peptides. Proc. 16th Eur. Pept. Symp., 1980, Scriptor, Copenhagen, 1981, pp. 706–710.
E. Giralt, M.-D. Ludevid, and E. Pedroso. Bioorg. Chem. 14:405–416 (1986).
W. P. Jencks and J. Carriulo. J. Biol. Chem. 234:1272–1279 (1959).
W. B. Melchior, Jr., and D. Fahrney. Biochemistry 9:251–258 (1970).
U. Klixbüll and H. Bundgaard. Arch. Pharm. Chem. Sci. Ed. 11:101–110 (1983).
R. Wolfenden and W. P. Jencks. J. Am. Chem. Soc. 83:4390–4393 (1961).
G. Grant, N. Ling, J. Rivier, and W. Vale. Biochemistry 11:3070–3073 (1972).
S. Wilk. Ann. N.Y. Acad. Sci. 553:252–264 (1989).
M. Orlowski and A. Meister. In P. D. Boyer (ed.), The Enzymes, Academic Press, New York, 1971, Vol. IV, pp. 123–151.
G. N. Abraham and D. N. Podell. Mol. Cell. Biochem. 38:181–190 (1981).
T. C. Friedman and S. Wilk. J. Neurochem. 46:1231–1239 (1986).
S. Wilk and E. K. Wilk. Ann. N.Y. Acad. Sci. 553:556–558 (1989).
S. Wilk. Life Sci. 39:1487–1492 (1986).
K. Bauer, P. Nowak, and H. Kleinkauf. Eur. J. Biochem. 118:173–176 (1981).
R. Lanzara, M. Liebman, and S. Wilk. Ann. N.Y. Acad. Sci. 553:559–562 (1989).
J. C. Dvorak and R. D. Utiger. J. Clin. Endocrinol. Metab. 44:582–585 (1977).
C. Hansch and A. Leo. Substituent Constants for Correlation Analysis in Chemistry and Biology, John Wiley & Sons, New York, 1979.
D. A. Brent, J. J. Sabatka, D. J. Minick, and D. W. Henry. J. Med. Chem. 26:1014–1020 (1983).
T. L. Hakfenschied and E. Tomlinson. Int. J. Pharm. 16:225–239 (1983).
H. Bundgaard, E. Falch, C. Larsen, G. L. Mosher, and T. J. Mikkelson. J. Pharm. Sci. 75:775–783 (1986).
S. Yokohama, K. Yamashita, H. Toguchi, J. Takeuchi, and N. Kitamori. J. Pharm. Dyn. 7:101–111 (1984).
S. Yokohama, T. Yoshioka, K. Yamashita, and N. Kitamori. J. Pharm. Dyn. 7:445–451 (1984).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bundgaard, H., Møss, J. Prodrugs of Peptides. 6. Bioreversible Derivatives of Thyrotropin-Releasing Hormone (TRH) with Increased Lipophilicity and Resistance to Cleavage by the TRH-Specific Serum Enzyme. Pharm Res 7, 885–892 (1990). https://doi.org/10.1023/A:1015933504191
Issue Date:
DOI: https://doi.org/10.1023/A:1015933504191