Abstract
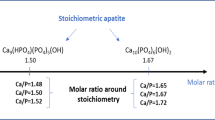
A new synthesis/processing method has been devised to produce magnesium/carbonate co-substituted hydroxyapatite ceramics that do not decompose to tricalcium phosphate (TCP) on sintering. Using this method, a series of magnesium/carbonate co-substituted hydroxyapatite (Mg/CO3–HA) compositions, containing between 0 and 0.35 wt % Mg and approximately 0.9 wt % CO3 were prepared. Sintering the Mg/CO3–HA compositions in a CO2/H2O atmosphere yields a single crystalline phase that appears to be identical to stoichiometric HA. In contrast, when the compositions were prepared in the absence of carbonate and were sintered in air, the phase composition was a biphasic mixture of HA and TCP e.g. for 0.25 wt % Mg substitution the phase composition was approximately 60%HA/40% TCP. Clearly, both the synthesis route and the processing (i.e. sintering) route are of importance in the production of a single-phase Mg/CO3–HA ceramic. Fourier transform infrared (FTIR) spectroscopy has indicated that the Mg/CO3–HA ceramics still contained carbonate groups after sintering at 1200 °C. Chemical analysis by X-ray fluorescence spectroscopy (XRF) and C–H–N analysis has shown that the cation/anion molar ratio (i.e. [Ca+Mg]/[P+C/2]) of the different compositions were 1.68(±0.01), which is equivalent to the Ca/P molar ratio of stoichiometric HA. Although the magnesium/carbonate co-substitution had a positive effect in preventing phase decomposition during sintering, it appeared to have a negative effect on the densification of the MgCO3–HA ceramics, compared to stoichiometric HA.
Similar content being viewed by others
References
Y. L. Liu, J. Schoenaers, K. De Groot, J. R. De Wijn and E. Schepers, J. Mater. Sci. Mater. in Med. 11 (2000) 711.
T. Kobayashi, S. Shingaki, T. Nakajima and K. Hanada, J. Long-Term Effects of Med. Impl. 3 (1993) 283.
A. Moroni, V. L. Caja, E. L. Egger, L. Trinchese and E. Y. S. Chao, Biomaterials 15 (1994) 926.
C. P. A. T. Klein, A. A. Driessen, K. De Groot and A. Van Den Hooff, J. Biomed. Mater. Res. 17 (1983) 769.
A. Bigi, G. Cojazzi, S. Panzavolta, A. Ripamonti, N. Roveri, M. Romanello, K. Noris Suarez and L. Moro, J. Inorg. Biochem. 68 (1997) 45-51.
F. C. M. Driessens, Bull Soc. Chem. Belg. 89 (1980) 663.
M. Kakei, H. Nakahara, N. Tamura, H. Itoh and M. Kumegawa, Ann. Anat. 179 (1997) 311.
A. Bigi, E. Foresti, R. Gregorinin, A. Ripamonti, N. Roveri and J. S. Shah, Calcif. Tissue Int. 50 (1992) 439.
R. Z. Legeros, R. Kijkowska, C. Bautista and J. P. Legeros, Conn. Tissue. Res. 33 (1995) 203.
J. D. B. Featherstone, I. Mayer, F. C. M. Driessens, R. M. H. Verbeeck and H. J. M. Heijligers, Calcif. Tissue Int. 35 (1983) 169.
R. N. Correia, M. C. F. MagalhÃes, P. A. A. P. Marques and A. M. R. Senos, J. Mat. Sci. Mater. in Med. 7 (1996) 501.
K. TÔnsuaadu, M. Peld, T. LeskelÄ, R. Mannonen, L. NiinistÖ and M. Veiderma, Thermochimica Acta 256 (1995) 55.
A. Yasukawa, S. Ouchi, K. Kandori and T. Ishikawa, J. Mater. Chem. 6 (1996) 1401.
I. Mayer, R. Schlam and J. D. B. Featherstone, J. Inorg. Biochem. 66 (1997) 1.
A. Bigi, G. Falini, E. Foresti, M. Gazzano, A. Ripamonti and N. Roveri, ibid. 49 (1993) 69.
K. Ishikawa, P. Ducheyne and S. Radin, J. Mater. Sci. Mater. in Med. 4 (1993) 165.
I. R. Gibson, I. U. Rehman, S. M. Best and W. Bonfield, ibid. 11 (2000) 533.
B. Dickens, L. W. Schroeder and W. E. Brown, J. Solid State Chem. 10 (1974) 232.
D. Clement, J. M. Tristan, M. Hamad, P. Roux and J. G. Heughebaert, ibid. 78 (1989) 271.
J. Ando, Bull. Chem. Soc. Japan 31 (1958) 201.
R. Z. Legeros, Prog. Crystal Growth Charact. 4 (1981) 1.
D. G. A. Nelson and J. D. B. Featherstone, Calc. Tiss. Int. 34 (1982) S69.
R. Z. Legeros, O. R. Trautz, J. P. Legeros and E. Klein, Bull. Soc. Chim. France (1968) 1712.
M. Vignoles, G. Bonel and R. A. Young, Calcif. Tissue Int. 40 (1987) 64.
M. Vignoles, G. Bonel, D. W. Holcomb and R. A. Young, ibid. 43 (1988) 33.
M. Okazaki, Biomaterials 12 (1991) 831.
M. Akao, H. Aoki and K. Kato, J. Mat. Sci. 16 (1981) 809.
I. R. Gibson and W. Bonfield, “Process for the Preparation of Magnesium and Carbonate Substituted Hydroxyapatite”, International Patent Application No. PCT/GB98/03817.
Idem., J. Biomed Mater. Res. 59 (2002) 697.
I. R. Gibson, S. Ke, S. M. Best and W. Bonfield, J. Mat. Sci. Mater. In Med. 12 (2001) 163.
PDF Card no. 9-432, ICDD, Newton Square, Pennsylvania, U.S.A.
A. C. Larson, R. B. Von Dreele and M. Lujan Jr., GSAS — Generalised Crystal Structure Analysis System, Neutron Scattering Centre, Los Alamos National Laboratory, California (1990).
I. Kay, R. A. Young and A. S. Posner, Nature 204 (1964) 1050.
I. Rehman and W. Bonfield, J. Mat. Sci. Mater. Med. 8 (1997) 1.
A. A. Campbell, M. Loroe and G. H. Nancollas, Colloids and Surfaces 54 (1991) 25.
A. SlÓsarczyk, E. Stobierska, Z. Paszkiewicz and M. Gawlicki, J. Am. Ceram. Soc. 79 (1996) 2539.
R. Z. Legeros, Nature 205 (1965) 403.
J. Barralet, PhD Thesis, University of London, UK (1995).
R. Z. Legeros, O. R. Trautz, E. Klein and J. P. Legeros, Experimentia 25 (1969) 5.
H. El Feki, C. Rey and M. Vignoles, Calc. Tiss. Int. 49 (1991) 269.
Y. Suwa, H. Banno, H. Saito, Y. Doi, T. Koda, M. Adachi and Y. Moriwaki, in “Bioceramics”, Vol. 6, edited by P. Ducheyne and D. Christiansen (Butterworth-Heinemann Ltd., Oxford, 1993) 381-386.
I. R. Gibson, S. M. Best and W. Bonfield (submitted J. Am. Ceram. Soc. 2001).
N. Senamaud, D. Bernache-Assollant, E. Champion, M. Heughebaert and C. Rey, Solid State Ionics 101–103 (1997) 1357.
Y. Doi, T. Koda, M. Adachi, N. Wakamatsu, T. Goto, H. Kamemizu, Y. Moriwaki and Y. Suwa, J. Biomed. Mater. Res. 29 (1995) 1451.
J. C. Merry, I. R. Gibson, S. M. Best and W. Bonfield, J. Mat. Sci. Mater. Med. 9 (1998) 779.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Gibson, I.R., Bonfield, W. Preparation and characterization of magnesium/carbonate co-substituted hydroxyapatites. Journal of Materials Science: Materials in Medicine 13, 685–693 (2002). https://doi.org/10.1023/A:1015793927364
Issue Date:
DOI: https://doi.org/10.1023/A:1015793927364