Abstract
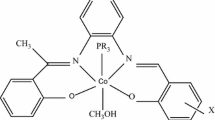
The Schiff base prepared by reacting (−)-(1R,2R)-1,2-cyclohexanediamine with 2-hydroxyacetophenone was used as a ligand for CoII and CuII. The coordination compounds were studied by u.v.–vis. absorption and by circular dichroism (c.d.) spectroscopy in solution. The complexes are four-coordinated in a slightly distorted square planar symmetry. The distortion from planarity is a main factor influencing the chiral surroundings of the metal ion. The d–d and c.t. transitions are consistent with the observed distortion, which arises from intramolecular interactions between the methyl groups attached to the Schiff base imine carbon and hydrogen atoms of the cyclohexane ring. The electrochemical properties of the CoII and CuII complexes were observed in MeCN but investigations revealed weaker oxygen activation than of CoII analogue with salicylaldehyde. The CuII complex is reduced in H2O to CuI which disproportionates to CuII and Cu0.
Similar content being viewed by others
References
C.P. Horowitz, S.E. Creager and R.W. Murray, Inorg. Chem., 29, 1006 (1990).
M. Gomes de F.T. and O.A.C. Anutunes, Catal. Lett., 38, 133 (1996).
J.M. Duprilot, F. Bediouni, J. Devynck, J.C. Folest and C. Bied-Charreton, J. Organometal. Chem., 286, 77 (1985).
C.E. Dahm, D.G. Peters, Anal. Chem., 66, 3117 (1994).
R.S. Downing and F.L. Urbach, J. Am. Chem. Soc., 91, 5977 (1969).
J. Llewellyn and T.N. Waters, J. Chem. Soc. Dalton Trans., 2639 (1960).
P. Gourec and M. Savy, Electrochim. Acta., 44, 2653 (1990).
S.K. Dhar, Inorg. Chim. Acta, 240, 609 (1995).
C.X. Cai, K.H. Xue, X.Y. Xu and Q.H. Luo, J. Appl. Electrochem., 27, 793 (1997).
E. Szłyk, S. Biniak and E. Larsen, J. Solid State Electrochem., 5, 211 (2001)
F. Galsbol, P. Steenbol and B.S. Sorensen, Acta Chem. Scand., 26, 962 (1972).
B. Bosnich, J. Am. Chem. Soc., 90, 627 (1968).
C.E. Schäffer, Pure Appl. Chem., 24, 361 (1970).
Bendix Ligeld V.078 Manual ed. University of Copenhagen.
A. Wojtczak, E. Szłyk, M. Jaskólski and E. Larsen, Acta Chem. Scand., 51, 274 (1997).
E. Szłyk, A. Surdykowski, M. Barwiołek and E. Larsen, Transition Met.Chem., 25, 670 (2000).
E. Szłyk, A. Wojtczak, E. Larsen, A. Surdykowski and J. Neumann, Inorg.Chim.Acta., 93, 239 (1999).
F. Beudiouni, E. Labbe, S. Gutierrez-Granados and J. Devynck, J. Electroanal.Chem., 301, 267 (1991).
R.R. Gagne, J.L. Allison, C.A. Kowal, W.S. Mialki, T.J. Smith and R.A. Walton, J.Am.Chem.Soc., 102, 1905 (1980).
A. Cinquanti, R. Cini, R. Seerbere and P. Zanello, J.Electroanal. Chem., 121, 301 (1981).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Szłyk, E., Biniak, S., Surdykowski, A. et al. Cyclovoltammetric and spectroscopic characterization of optically active cobalt(II) and copper(II) complexes with the Schiff base derived from (N,N′)-(1R,2R)-(−)-cyclohexylenediamine and 2-hydroxyacetophenone. Transition Metal Chemistry 27, 501–505 (2002). https://doi.org/10.1023/A:1015600414398
Issue Date:
DOI: https://doi.org/10.1023/A:1015600414398