Abstract
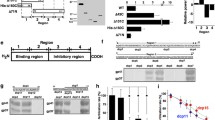
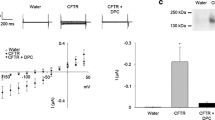
We have developed a coupled Xenopus oocyte expression system for evaluating the functional effects of mutations in known or suspected adhesion molecules, which allows for a very rapid assessment of intercellular adhesion. As a model protein, we first used Protein zero (Po), an adhesion molecule that mediates self-adhesion of the Schwann cell plasma membrane to form compact myelin in the mammalian PNS. A wide variety of mutations in Po cause certain human peripheral neuropathies, such as the Charcot-Marie-Tooth disease (CMT) type 1B and Dejerine-Sottas syndrome (DSS). After wild-type Po mRNA is injected, the protein is synthesized and correctly targeted to the oocyte cell surface. When two oocytes are paired, wild-type Po redistributes and concentrates at the cell-cell apposition region, and by electron microscopy, the oocyte pairs show close cell-cell appositions and are devoid of the microvilli that are observed in uninjected oocyte pairs. These are hallmark features of highly adhesive cell:cell interfaces. Several point mutations in Po were engineered, corresponding to the molecular defects in the CMT type 1B or DSS. The proteins encoded by these mutations reached the cell surface but failed to concentrate at the oocyte interface. Po carrying a point mutation that is found in DSS is not targeted on the plasma membrane and fail to accumulate at the cell-cell contact site.
Similar content being viewed by others
REFERENCES
D'Urso, D., Brophy, P. J., Staugaitis, S. M., Gillespie, C. S., Frey, A. B., Stempak, J. G., and Colman, D. R. 1990. Protein zero of peripheral nerve myelin: biosynthesis, membrane insertion, and evidence for homotypic interaction. Neuron 4:449–460.
Doyle, J. P., Stempak, J. J., Cowin, P., Colman, D. R., and D'Urso, D. 1995. Protein zero, a nervous system adhesion molecule, triggers epithelial reversion in host carcinoma cells. J. Cell Biol. 131:465–482.
Filbin, M. T., Walsh, F. S., Trapp, B. D., Pizzey, J. A., and Tennekoon, G. I. 1990. Role of myelin P0 protein as a homophilic adhesion molecule. Nature 344:871–872.
Nagafuchi, A. and Takeichi, M. 1988. Cell binding function of E-cadherin is regulated by the cytoplasmic domain. Embo J. 7:3679–3684.
Brackenbury, R., Thiery, J. P., Rutishauser, U., and Edelman, G. M. 1977. Adhesion among neural cells of the chick embryo. I. An immunological assay for molecules involved in cell-cell binding. J. Biol. Chem. 252:6835–6840.
Doyle, J. P. and Colman, D. R. 1993. Glial-neuron interactions and the regulation of myelin formation. Curr. Opin. Cell Biol. 5:779–785.
Lai, C., Brow, M. A., Nave, K. A., Noronha, A. B., Quarles, R. H., Bloom, F. E., Milner, R. J., and Sutcliffe, J. G. 1987. Two forms of 1B236/myelin-associated glycoprotein, a cell adhesion molecule for postnatal neural development, are produced by alternative splicing. Proc. Natl. Acad. Sci. USA 84:4337–4341.
Lemke, G., Lamar, E., and Patterson, J. 1988. Isolation and analysis of the gene encoding peripheral myelin protein zero. Neuron 1:73–83.
Schneider Schaulies, J., von Brunn, A., and Schachner, M. 1990. Recombinant peripheral myelin protein Po confers both adhesion and neurite outgrowth-promoting properties. J. Neurosci. Res. 27:286–297.
Giese, K. P., Martini, R., Lemke, G., Soriano, P., and Schachner, M. 1992. Mouse P0 gene disruption leads to hypomyelination, abnormal expression of recognition molecules, and degeneration of myelin and axons. Cell 71:565–576.
Hayasaka, K., Himoro, M., Sato, W., Takada, G., Uyemura, K., Shimizu, N., Bird, T. D., Conneally, P. M., and Chance, P. F. 1993. Charcot-Marie-Tooth neuropathy type 1B is associated with mutations of the myelin P0 gene. Nat. Genet. 5:31–34.
Hayasaka, K., Ohnishi, A., Takada, G., Fukushima, Y., and Murai, Y. 1993. Mutation of the myelin P0 gene in Charcot-Marie-tooth neuropathy type 1. Biochem. Biophys. Res. Commun. 194:1317–1322.
Hayasaka, K., Himoro, M., Sawaishi, Y., Nanao, K., Takahashi, T., Takada, G., Nicholson, G. A., Ouvrier, R. A., and Tachi, N. 1993. De novo mutation of the myelin P0 gene in Dejerine-Sottas disease (hereditary motor and sensory neuropathy type III). Nat. Genet. 5:266–268.
Warner, L. E., Hilz, M. J., Appel, S. H., Killian, J. M., Kolodry, E. H., Karpati, G., Carpenter, S., Watters, G. V., Wheeler, C., Witt, D., Bodell, A., Nelis, E., Van Broeckhoven, C., and Lupski, J. R. 1996. Clinical phenotypes of different MPZ (P0) mutations may include Charcot-Marie-Tooth type 1B, Dejerine-Sottas, and congenital hypomyelination. Neuron 17:451–460.
Mishina, M., Kurosaki, T., Tobimatsu, T., Morimoto, Y., Noda, M., Yamamoto, T., Terao, M., Lindstrom, J., Takahashi, T., Kuno, M., and et al. 1984. Expression of functional acetylcholine receptor from cloned cDNAs. Nature 307:604–608.
Werner, R., Miller, T., Azarnia, R., and Dahl, G. 1985. Translation and functional expression of cell-cell channel mRNA in Xenopus oocytes. J. Membr. Biol. 87:253–268.
Dahl, G., Miller, T., Paul, D., Voellmy, R., and Werner, R. 1987. Expression of functional cell-cell channels from cloned rat liver gap junction complementary DNA. Science 236:1290–1293.
Paul, D. L., Ebihara, L., Takemoto, L. J., Swenson, K. I., and Goodenough, D. A. 1991. Connexin46, a novel lens gap junction protein, induces voltage-gated currents in nonjunctional plasma membrane of Xenopus oocytes. J. Cell Biol. 115:1077–1089.
Lemke, G. and Axel, R. 1985. Isolation and sequence of a cDNA encoding the major structural protein of peripheral myelin. Cell 40:501–508.
Liman, E. R., Tytgat, J., and Hess, P. 1992. Subunit stoichiometry of a mammalian K1 channel determined by construction of multimeric cDNAs. Neuron 9:861–871.
Dumont, J. N. 1972. Oogenesis in Xenopus laevis (Daudin). I. Stages of oocyte development in laboratory maintained animals. J. Morphol. 136:153–179.
Kulkens, T., Bolhuis, P. A., Wolterman, R. A., Kemp, S., te Nijenhuis, S., Valentijn, L. J., Hensels, G. W., Jennekens, F. G., de Visser, M., Hoogendijk, J. E., and et al. 1993. Deletion of the serine 34 codon from the major peripheral myelin protein P0 gene in Charcot-Marie-Tooth disease type 1B. Nat. Genet. 5:35–39.
Nelis, E., Timmerman, V., De Jonghe, P., Vandenberghe, A., Pham-Dinh, D., Dautigny, A., Martin, J. J., and Van Broeckhoven, C. 1994. Rapid screening of myelin genes in CMT1 patients by SSCP analysis: identification of new mutations and polymorphisms in the P0 gene. Hum. Genet. 94:653–657.
Swenson, K. I., Jordan, J. R., Beyer, E. C., and Paul, D. L. 1989. Formation of gap junctions by expression of connexins in Xenopus oocyte pairs. Cell 57:145–155.
Zhang, K. and Filbin, M. T. 1994. Formation of a disulfide bond in the immunoglobulin domain of the myelin P0 protein is essential for its adhesion. J. Neurochem. 63:367–370.
Zhang, K. and Filbin, M. T. 1998. Myelin Po protein mutated at Cys21 has a dominant-negative effect on adhesion of wild type Po. J. Neurosci. Res. 53:1–6.
Gao, Y., Li, W. and Filbin, M. T. 2000. Acylation of myelin Po protein is required for adhesion. J. Neurosci. Res. 60:704–713.
Munchberg, F. E., Spieker, T. P., Joos, T. O., and Hausen, P. 1997. A paired oocyte adhesion assay reveals the homophilic binding properties of the Xenopus maternal cadherins, XB/Uand EP-cadherin. Mech. Dev. 64:87–94.
Shapiro, L., Doyle, J. P., Hensley, P., Colman, D. R., and Hendrickson, W. A. 1996. Crystal structure of the extracellular domain from P0, the major structural protein of peripheral nerve myelin. Neuron 17:435–449.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yoshida, M., Colman, D.R. Rapid Functional Analysis in Xenopus Oocytes of Po Protein Adhesive Interactions. Neurochem Res 26, 703–712 (2001). https://doi.org/10.1023/A:1010999622760
Issue Date:
DOI: https://doi.org/10.1023/A:1010999622760