Abstract
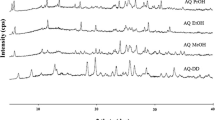
The crystal structure of the methanol solvate (empirical formula: 2C20H30N2O5·3CH3OH) of a new dipeptide sweetener, neotame (N-(3,3-dimethylbutyl)-L-α-aspartyl-L-phenylalanine 1-methyl ester), has been determined. Crystal data: a = 9.8989(1), b = 18.1331(1), c = 27.5725(1) Å, orthorhombic, space group P212121, with Z = 4. Each unit cell includes 8 neotame and 12 methanol molecules. Disorder exists in one neotame molecule and one methanol molecule. The crystals were characterized by the following techniques: hot-stage microscopy (HSM), Karl-Fischer titrimetry (KFT), powder X-ray diffractometry (PXRD), differential scanning calorimetry (DSC), thermogravimetry (TGA), 13C solid-state nuclear magnetic resonance (SSNMR) spectroscopy. Under HSM at a heating rate of 10°C/min in silicone oil, the sample melts at 64–84°C and liberates bubbles at 71–86°C. DSC in open pans shows two overlapping endotherms at 56 and 71°C, probably due to melting and desolvation, respectively. TGA in open pans shows 5.9% weight loss due to desolvation below 70°C. Under house vacuum (23 mm Hg) over phosphorus pentoxide at 23°C, the methanol solvate produces pure amorphous anhydrate, which converts to crystalline neotame monohydrate in the presence of moisture.
Similar content being viewed by others
References
Brittain, H.G. Ed., Polymorphism in Pharmaceutical Solids; Marcel Dekker: New York; 1999.
Threlfall, T.L. Analyst 1995, 120, 2435.
Zhu, H.J.; Yuen, C.; Grant, D.J.W. Int. J. Pharm. 1996, 135, 151.
Zhu, H.J.; Grant, D.J.W. Int. J. Pharm. 1996, 139, 33.
Khankari, R.K.; Grant, D.J.W. Thermochim. Acta 1995, 248, 61.
U.S. Patent Number 5510805.
Prakash, I.; Bishay, I.; Schroeder, S. Synth. Commun. 1999, 24, in press.
U.S. Patent Number 5728862.
Padden, B.E.; Zell, M.T.; Dong, Z.; Schroeder, S.A.; Grant, D.J.W.; Munson, E.J. Anal. Chem. 1999, 71, 3325.
Leung, S.S.; Padden, B.E.; Munson, E.J.; Grant, D.J.W. J. Pharm. Sci. 1998, 87, 508.
Leung, S.S.; Padden, B.E.; Munson, E.J.; Grant, D.J.W. J. Pharm. Sci. 1998, 87, 501.
Leung, S.S.; Grant, D.J.W. J. Pharm. Sci. 1997, 86, 64.
Mattern, R.H.; Amino, Y.; Benedetti, E.; Goodman, M. J. Peptide Res. 1997, 50, 286.
Goodman, M.; Mattern, R.H.; Santini, P.G.A.; Iacovino, R.; Saviano, M.; Benedetti, E. J. Peptide Sci. 1998, 4, 229.
SHELXTL-Plus V5.0; Siemens Industrial Automation, Inc: Madison, WI.
Blessing, R.H. Acta Crystallogr. 1995, A51, 33.
Pines, A.; Gibby, M.G.; Waugh, J.S. J. Chem. Phys. 1973, 59, 569.
Andrew, E.R. Prog. NMR Spec. 1971, 8, 1.
Dixon, W.T.; Schaefer, J.; Sefcik, M.D.; Stejskal, E.O.; Mckay, R.A. J. Magn. Res. 1982, 49, 341.
Spek, A.L. Acta Crystallogr. 1990, A46, C34.
Wink, D.J.; Schroeder, S.A.; Prakash, I.; Lam K.-C.; Rheingold, A.L. Acta Crystallogr. 1999, C55, 1365.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Dong, Z., Young, V.G., Padden, B.E. et al. Crystal structure and physical characterization of neotame methanol solvate. Journal of Chemical Crystallography 29, 967–975 (1999). https://doi.org/10.1023/A:1009542515709
Issue Date:
DOI: https://doi.org/10.1023/A:1009542515709