Abstract
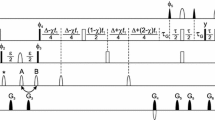
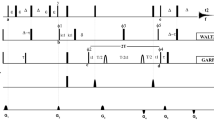
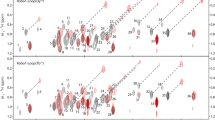
A triple resonance pulse scheme is presented for recording 13Cα-1Hα one-bond dipolar couplings in 15N, 13C labeled proteins. HNCO correlation maps are generated where the carbonyl chemical shift is modulated by the 13Cα-1Hα coupling, with the two doublet components separated into individual data sets. The experiment makes use of recently described methodology whereby the protein of interest is dissolved in a dilute solution of bicelles which orient above a critical temperature, thus permitting measurement of significant couplings (Tjandra and Bax, 1997a). An application to the protein ubiquitin is described.
Similar content being viewed by others
References
Bax, A., and Ikura, M. (1991) J. Biomol. NMR, 1, 99–104.
Bodenhausen, G., and Ernst, R. R. (1981) J. Magn. Reson., 45, 367–373.
Boyd, J., and Scoffe, N. (1989) J. Magn. Reson., 85, 406–413.
Delaglio, F., Grzesiek, S., Vuister, G. W., Zhu, G., Pfeifer, J., and Bax, A. (1995) J. Biomol. NMR, 6, 277–293.
Freeman, R., Kempsell, S. P., and Levitt, M. H. (1980) J. Magn. Reson., 38, 453–479.
Garrett, D. S., Powers, R., Gronenborn, A. M., and Clore, G. M. (1991) J. Magn. Reson., 95, 214–220.
Grzesiek, S., and Bax, A. (1993) J. Biomol. NMR, 3, 185–204.
Kay, L. E. (1993) J. Am. Chem. Soc., 115, 2055–2056.
Kay, L. E., Ikura, M., Tschudin, R., and Bax, A. (1990) J. Magn. Reson., 89, 496–514.
Kay, L. E., Keifer, P., and Saarinen, T. (1992) J. Am. Chem. Soc., 114, 10663–10665.
Logan, T. M., Olejniczak, E. T., Xu, R. X., and Fesik, S. W. (1993) J. Biomol. NMR, 3, 225–231.
Marion, D., Ikura, M., Tschudin, R., and Bax, A. (1989) J. Magn. Reson., 85, 393–399.
McCoy, M., and Mueller, L. (1992) J. Am. Chem. Soc., 114, 2108–2110.
Muhandiram, D. R., and Kay, L. E. (1994) J. Magn. Reson. Ser. B., 103, 203–216.
Palmer, A. G., and Case, D. A. (1992) J. Am. Chem. Soc., 114, 9059–9067.
Patt, S. L. (1992) J. Magn. Reson., 96, 94–102.
Sanders, C. R., Hare, B. J., Howard, K. P., and Prestegard, J. H. (1994) Prog. NMR. Spectrosc., 26, 421–444.
Sanders, C. R., and Schwonek, J. P. (1992) Biochemistry, 31, 8898–8905.
Schleucher, J., Sattler, M., and Griesinger, C. (1993) Angew. Chem. Int. Ed. Engl., 32, 1489–1491.
Shaka, A. J., Keeler, J., Frenkiel, T., and Freeman, R. (1983) J. Magn. Reson., 52, 335–338.
Sorensen, M. D., Meissner, A., and Sorensen, O. W. (1997) J. Biomol. NMR, 10, 181–186.
Tjandra, N., and Bax, A. (1997a) Science, 278, 1111–1114.
Tjandra, N., and Bax, A. (1997b) J. Magn. Reson., 124, 512–515.
Tjandra, N., Feller, S. E., Pastor, R. W., and Bax, A. (1995) J. Am. Chem. Soc., 117, 12562–12566.
Tjandra, N., Grzesiek, S., and Bax, A. (1996) J. Am. Chem. Soc., 118, 6264–6272.
Tjandra, N., Omichinski, J. G., Gronenborn, A. M., Clore, G. M., and Bax, A. (1997) Nat. Struct. Biol., 4, 732–738.
Tolman, J. R., Flanagan, J. M., Kennedy, M. A., and Prestegard, J. H. (1995) Proc. Natl. Acad. Sci., 92, 9279–9283.
Tolman, J. R., Flanagan, J. M., Kennedy, M. A., and Prestegard, J. H. (1997) Nature Structure Biology, 4, 292–297.
Tolman, J. R., and Prestegard, J. H. (1996a) J. Magn. Reson. Ser. B., 112, 269–274.
Tolman, J. R., and Prestegard, J. H. (1996b) J. Magn. Reson. Ser. B., 112, 245–252.
Vijay-Kumar, S., Bugg, C. E., and Cook, W. J. (1987) J. Mol. Biol., 194, 531–544.
Vuister, G. W., and Bax, A. (1992) J. Magn. Reson., 98, 428–435.
Vuister, G. W., Delaglio, F., and Bax, A. (1992) J. Am. Chem. Soc., 114, 9674–9675.
Wüthrich, K. (1986) NMR of Proteins and Nucleic Acids, John Wiley and Sons, New York.
Yamazaki, T., Lee, W., Arrowsmith, C. H., Muhandiram, D. R., and Kay, L. E. (1994) J. Am. Chem. Soc., 116, 11655–11666.
Yang, D., and Nagayama, K. (1996) J. Magn. Reson. Ser. A, 118, 117–121.
Zhu, G., and Bax, A. (1990) J. Magn. Reson., 90, 405–410.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Yang, D., Tolman, J.R., Goto, N.K. et al. An HNCO-based Pulse Scheme for the Measurement of 13Cα-1Hα One-bond Dipolar couplings in 15N, 13C Labeled Proteins. J Biomol NMR 12, 325–332 (1998). https://doi.org/10.1023/A:1008223017233
Issue Date:
DOI: https://doi.org/10.1023/A:1008223017233