Abstract
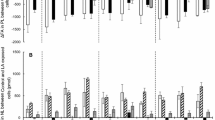
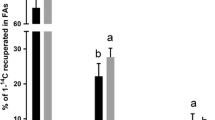
Atlantic salmon (Salmo salar) were fed diets containing fish oil supplemented with 22:6n-3 (FO diet) or linseed oil supplemented with 20:5n-3 (LO diet) for 6\(\frac{1}{2}\) months. The effects of these diets, both containing about 36% n-3 fatty acids, on the esterification, desaturation and elongation of [1-14C] 18:2n-6 and [1-14C] 18:3n-3 were investigated in isolated hepatocytes. The percentages of radioactivity which was esterified from [1-14C] 18:2n-6 or [1-14C]18:3n-3 into total lipids, were approximately 20% lower in hepatocytes from fish fed the FO diet than in hepatocytes from fish fed the LO diet. The percentages of radioactivity esterified in both groups were further reduced when 0.1 mM unlabelled 22:6n-3 was added to the incubation. The percentage of desaturation and elongation products formed from [1-14C] 18:2n-6 was twice as high in hepatocytes from salmon fed the FO diet as it was in hepatocytes from fish fed the LO diet. The ratio of 18:2n-6 to 18:3n-3 was five times higher in the FO diet, and this probably promoted the conversion of 18:2n-6 to longer chain n-6 fatty acids. When 0.1mM unlabelled 22:6n-3 was added to the incubation medium, the percentages of desaturation and elongation products formed were unchanged. Thus, a high level of 22:6n-3 in the diet is apparently not inhibiting the conversion of 18:2n-6 to 20:4n-6, as long as the amount of 18:2n-6 present is substantially higher than that of 18:3n-3. No desaturation and elongation products were recovered from the phospholipids of hepatocytes incubated with [1-14C] 18:3n-3 in any of the groups. However, the `dead end' elongation product 20:3n-3 was found in the triacylglycerol fraction, and the percentage of this fatty acid increased when 22:6n-3 was added to the incubation medium.
Similar content being viewed by others
References
Austreng, E., Storebakken, T. and Åsgård, T. 1987. Growth rate estimates for cultured Atlantic salmon and rainbow trout. Aquaculture 60: 157-160.
Ackmann, R.G. and Takeuchi, T. 1986. Comparison of fatty acids and lipids of smolting hatchery-fed and wild Atlantic salmon. Lipids 21: 117-120.
Bell, J.G., Dick, J.R., MC Vicar, A.H., Sargent, J.R. and Thompson, K.D. 1993. Dietary sunflower, linseed and fish oils affect phospholipid fatty acid composition, development of cardiac lesions, phospholipase activity and eicosanoid production in Atlantic salmon. Prostaglandins, Leukotriens and Essential Fatty acids 49: 665-673.
Bell, J.G., Ghioni, C. and Sargent, J.R., 1994. Fatty acid composition of 10 freshwater invertebrates which are natural food organisms of Atlantic salmon parr (Salmo salar): a comparison with commercial diets. Aquaculture 128: 301-313.
Bell, J.G., Ashton, C.J., Weitzel, B.R., Dick, J.R and Sargent, J.R. 1996. Dietary lipid affects phospholipid fatty acid compositions, eicosanoid production and immune function in Atlantic salmon (Salmo salar). Prostaglandins, Leukotriens and Essential Fatty Acids 54:173-182.
Bell, J.G., Tocher, D.R., Farndale, B.M., Cox, D.I., McKinney, R.W. and Sargent, J.R. 1997. The effect of dietary lipid on polyunsaturated fatty acid metabolism in Atlantic salmon (Salmo salar) undergoing parr-smolt transformation. Lipids 32: 515-525.
Brenner, R.R. De Thomas, M.E. and Peluffo, R.O. 1975. Effect of polyunsaturated fatty acids on the desaturation in vitro of palmitic acid, stearic acid, linoleic acid and linolenic acid. J.Biol. Chem. 241: 5213-5219.
Brenner, R.R. 1981a. Early effects of essential fatty acid deficiency on the structure and enzymatic activity of rat liver microsomes. Progr. Lipid Res. 20: 41-47.
Brenner, R.R. 1981b. Nutritional and hormonal factors influencing desaturation of essential fatty acids in animals. Progr. Lipid Res. 20: 41-47.
Buzzi, M., Henderson, R.J. and Sargent, J.R. 1996. The desaturation and elongation of linolenic acid and eicosapentaenoic acid by hepatocytes and liver microsomes from rainbow trout (Onchorhynchus mykiss) fed diets containing fish oil or olive oil. Biochem. Biophys. Acta. 1299: 235-244.
Buzzi, M., Henderson, R.J. and Sargent, J.R. 1997 Biosynthesis of docosahexaenoic acid in trout hepatocytes proceeds via 24-carbon intermediates. Comp. Biochem. Physiol. 116B:263-267.
Cao, J.-M., Blond, J.P., Juaneda, P., Durand, G. and Bezard, J. 1995. Effects of low levels of dietary fish oils on fatty acid desaturation and tissue fatty acids in obese and lean rats. Lipids 30: 825-832.
Cinti, D.L., Cook, L., Nagi, M.N. and Suneja, S.K. 1992. The fatty acid chain-elongation system of mammalian endoplasmatic reticulum. Progr. Lipid. Res. 31: 1-51.
Christiansen, E.N., Lund, J.S., Rørtveit, T. and Rustan, A.C. 1991. Effect of dietary n-3 and n6 fatty acids on fatty acid desaturation in rat liver. Biochim. Biophys. Acta 1082: 57-62.
Dannevig, B.H. and Berg, T. 1985. Endocytosis of galactoseterminated glycoproteins by isolated liver cells of rainbow trout. Comp. Biochem. Physiol. 82B: 683-688.
Folch, A.C., Leed, M. and Sloane-Stanley, G.M. 1957. A simple method for isolation and purification of total lipids from animal tissues.J. Biol. Chem. 226: 497-509.
Hansen, H. 1994. New biological and clinical roles for the n-6 and n-3 fatty acids. Nutr. Rev. 52: 162-167.
Henderson, R.J. 1996. Fatty acid metabolism in freshwater fish with particular reference to polyunsaturated fatty acids. Arch. Anim. Nutr. 49: 5-22.
Leger, C. and Fremont, L. 1981. Fatty acid composition of lipids in the trout (Salmo gairdneri). Comp. Biochem. Biophys. 69B: 99-105.
Lowry, O.H., Rosebrough, N.J., Farr, A.L.and Randall, R.J. 1951. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193: 265-275.
Mason, M.E. and Waller, G.R. 1964. Dimethoxypropane induces transesterification of fats and oils in preparation of methylesters for gas chromatographic analysis. Anal. Chem. 36: 583.
Narce, M., Gresti, J. and Bezard, J. 1988. Method for evaluating the bioconversion of radioactive polyunsaturated fatty acids by use of reversed-phase liquid chromatography. J.Chrom. 448: 249-264.
Owen, J.M., Adron, J.W., Middleton, C. and Cowey, C.B. 1975. Elongation and desaturation of dietary fatty acids in turbot Scophthalmus maximus L., and rainbow trout Salmo gairdneri. Rich. Lipids 10: 528-531.
Røsjø, C., Berg, T., Manum, K., Gjøen, T., Magnusson, S. and Thomassen, M.S. 1994. Effects of temperature and dietary n-3 and n-6 fatty acids on endocytic processes in isolated rainbow trout (Onchorhynchus mykiss, Walbaum) hepatocytes. Fish Physiol. Biochem. 13: 119-132.
Ruyter, B. and Thomassen M.S. 1999. Metabolism of n-3 and n-6 fatty acids in Atlantic salmon liver: Stimulation by essential fatty acid deficiency. Lipids 34: 1167-1176.
Sargent, J.R., Bell, J.G., Bell, M.V., Henderson, R.J. and Tocher, D.R. 1995. Requirement criteria for essential fatty acids. J. Appl. Ichthyol. 11: 183-198.
Sargent, J.R. 1997. Fish oils and human diet. Br. J. Nutr. 78: 5-13.
Seglen, P.O. 1976. Preparation of isolated rat liver cells. Meth. Cell Biol. 13: 29-59.
Sprecher, H. 1993 A reevaluation of the pathway for biosynthesis of 4,7,10,13,16,19 docosahexaenoic acid.Omega-3 News 7: 1-3.
Takeuchi, T., Toyota, M., Satoh, S. and Watanabe, T. 1990. Requirement of juvenile red sea bream (Pagrus major) for eicosapentaenoic and docosahexaenoic acids. Nippon Suisan Gakkaishi 56: 1263-1269.
Tocher, D.R., Bell, J.G., Dick, J.R. and Sargent, J.R. 1997. Fatty acyl desaturation in isolated hepatocytes from Atlantic salmon (Salmo salar): Stimulation by dietary borage oil containing-linolenic acid. Lipids 32: 1237-1247.
Ulmann, L., Bouziane, M., Mimouni, V., Belleville, J., and Poisson, J.-P. 1992. Relationship between rat liver 16-and 15-desaturase activities and fatty acid composition: Comparative effects of coconut and salmon oils during protein restriction. J. Nutr. Biochem. 3: 188-183.
Voss, A., Reinhart, M., Sankarappa, S. and Sprecher, H. 1991. The metabolism of 7,10,13,16,19 docosapentaenoic acid to 4,7,10,13,16,19 docosahexaenoic acid in rat liver is independent of a ?4-desaturase. J. Biol. Chem. 266: 19995-20000.
Yu, T.C. and Sinnhuber, R.O. 1976. Growth response of rainbow trout to dietary ?3 and ?6 fatty acids. Aquaculture 8: 309-317.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ruyter, B., Røsjø, C., Måsøval, K. et al. Influence of dietary n-3 fatty acids on the desaturation and elongation of [1-14C] 18:2 n-6 and [1-14C] 18:3 n-3 in Atlantic salmon hepatocytes. Fish Physiology and Biochemistry 23, 151–158 (2000). https://doi.org/10.1023/A:1007893317923
Issue Date:
DOI: https://doi.org/10.1023/A:1007893317923