Abstract
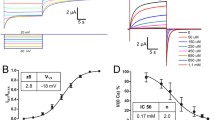
Voltage-dependent L-type Ca2+ channels form highly selective pores for Ca2+ ions in the membranes of excitable cells. We investigated the functional role of negatively charged residues, within or near the selectivity region, in ion permeation of a human cardiac L-type Ca2+ channel. Glutamates in each of the four repeats, and an aspartate in repeat IV, were substituted with positively charged lysine. Wild-type and mutant Ca2+ channels were expressed in Xenopus oocytes. Block by Ca2+ and Mg2 of inward Li+ currents through the channels was used to assess the effects of amino acid substitutions on high-affinity divalent cation binding. The rank order of IC50's for Ca2+ block of ILi was: E677K > E1086K > E334K > E1387K > D1391K > wild-type. The order of IC50's for Mg2+ block of ILi indicated differential involvement of the same residues in Mg2+ binding: E1387K > E334K > E1086K > E677K > D1391K ≅ wild-type. Mutants E1387K and D1391K effectively permeated Ba2+, but exhibited a decreased single-channel conductance. The unitary current amplitude carried by Na+, in the absence of external divalent cations, was slightly decreased in the E1387K mutant but not in the D1391K mutant. The results confirm that each of the four glutamates participate unequally in high-affinity Ca2+ binding. Additionally, our results indicate that these glutamate residues participate in Mg2+ binding. The glutamate at position 1387 may be only peripherally involved in the formation of a high-affinity Ca2+ -binding site but is central to a Mg2+ binding site accessible from the external side of the pore. The aspartate at position 1391 is most likely located just external to the selectivity region. (Mol Cell Biochem 166: 125-134, 1997)
Similar content being viewed by others
References
Guy HR, Seetharamulu P: Molecular model of the action potential sodium channel. Proc Natl Acad Sci USA 83: 508–512, 1986
Jan LY, Jan YN: Voltage-sensitive ion channels. Cell 56: 13–25, 1989
Tomaselli GF, Backx PH, Marban E. Molecular basis of permeation in voltage-gated ion channels. Circ Res 72: 491–496, 1993
Hartmann HA, Kirsch GE, Drewe JA, Taglialatela M, Joho RH, Brown AM: Exchange of conduction pathways between two related K+ channels. Science 251: 942–944, 1991
MacKinnon R, Yellen G: Mutations affecting TEA blockade and ion permeation in voltage-activated K+ channels. Science 250: 276–279, 1990
Yellen G, Jurman ME, Abramson T, MacKinnon R: Mutations affecting internal TEA blockade identify the probable poreforming region of a K channel. Science 251: 939–942, 1991
Yool AF, Schwartz TL: Alteration of ionic selectivity of a K channel by mutation of the H5 region. Nature 349: 700–704, 1991
Heinemann SH, Terlau W, Stühmer W, Imoto K, Numa S: Calcium channel characteristics conferred on the sodium channel by single mutations. Nature 356: 441–443, 1992
Noda M, Suzuki H, Numa S, Stühmer W: A single point mutation confers tetrodotoxin and saxitoxin sensitivity on the sodium channel II. FEBS Lett 259: 213–216, 1989
Terlau H, Heinemann S, Stuhmer W, Pusch M, Conti F, Imoto K, Numa S: Mapping the site of block by tetrodotoxin and saxitoxin of sodium channel II. FEBS Lett 293: 93–96, 1991
Guy HR, Durell SR: Models of the outer vestibules and selectivity filters of Na+, Ca2+, and K+ channels. (Abstract) Biophys J 66: A248, 1994
Schetz JA, Anderson PAV: A reevaluation of the structure in the pore region of voltage-activated cation channels. Biol Bull 185: 462–466, 1994
Mikala G, Bahinski A, Yatani A, Tang S, Schwartz A: Functional asymmetry of the ion permeation of a human L-type cardiac calcium channel. FEBS Lett 335: 265–269, 1993
Tang S, Mikala G, Bahinski A, Yatani A, Varadi G, Schwartz A: Molecular localization of ion selectivity sites within the pore of a human L-type cardiac calcium channel. J Biol Chom 268: 13026–13029, 1993
Yatani A, Bahinski A, Mikala G, Yamamoto S, Schwartz A: Single amino acid substitutions within the ion permeation pathway alter single-channel conductance of the human L-type cardiac Ca2+ channel. Circ Res 75: 315–323, 1994
Parent L, Gopalakrishnan M: Glutamate substitution in repeat IV alters divalent and monovalent cation permeation in the heart Ca2+ channel. Biophys J 69: 1801–1813, 1995
Ellinor PT, Wang J, Sather WA, Zhang J-F, Tsien RW: Ca2+ channel selectivity at a single locus for high-affinity Ca2+ interactions. Neuron 15: 1121–1132, 1995
Yang J, Ellinor PT, Sather WA, Zhang J-F, Tsien RW: Molecular determinants of Ca2+ selectivity and ion permeation in L-type Ca2+ channels. Nature 366: 158–161, 1993
Kuo C-C, Hess P: Block of the L-type Ca2+ channel pore by external and internal Mg2+ in rat phaeochromocytoma cells. J Physiol 466: 683–706, 1993
Green WN, Anderson OS: Surface charges and ion channel function. Annu Rev Physiol 53: 341–359, 1991
Cai M, Jordan PC: How does vestibule surface charge affect ion conduction and toxin binding in a sodium channel? Biophys J 57: 883–891, 1990
Dani JA: Ion-channel entrances influence permeation: net charge, size, shape and binding considerations. Biophys J 49: 607–618, 1986
Schultz D, Mikala G, Yatani A, Engle DB, Iles DF, Segers B, Sinke RJ, Olde Weghuis D, Klockner U, Wakamori M, Wang J, Melvin D, Varadi G, Schwartz A: Cloning, chromosomal localization and functional expression of the α1 subunit of the L-type voltage-dependent calcium channel from normal human heart. Proc Natl Acad Sci USA 90: 6228–6232, 1993
Ellis SB, Williams MR, Ways R, Brenner R, Sharp AH, Hewing AT, Campbell KP, McKenna E, Koch WJ, Hui A, Schwartz A, Harpold MM: Sequence and expression of mRNAs encoding the alphal and alpha2 subunits of a DHP-sensitive calcium channel. Science 241: 1661–1664, 1988
Powers PA, Liu S, Hogan K, Gregg RG: Skeletal muscle and brain isoforms of a β subunit of human voltage-dependent calcium channels are encoded by a single gene. J Biol Chem 267: 22967–22972, 1992
Collin T, Wang J, Nargeot J, Schwartz A: Molecular cloning of three isoforms of the L-type voltage-dependent calcium channel β subunit from normal human heart. Circ Res 72: 1337–1344, 1993
Fabiato A, Fabiato F: Calculator program for computing the composition of the solutions containing multiple metals and ligands used for experiments in skinned muscle cells. J Physiol (Paris) 75: 463–505, 1979
Yatani A, Okaba K, Birnbaumer L, Brown AM: Detergents, dimeric G, and eicosanoid pathways to muscarini channels. Am J Physiol 258: H1507-H1514, 1990
Matsuda H: Sodium conductance in calcium channels of guinea-pig ventricular cells induced by removal of external calcium ions. Pflügers Arch 407: 465–475, 1986
Hess P: Calcium channels in vertebrate cells. Ann Rev Neurosci 13: 337–356, 1990
Fraústo Da Silva JJR, Williams RJP: The Biological Chemistry of the Elements. Clarendon, Oxford, 1991
Kuo C-C, Hess P: Characterization of the high-affinity Ca2+-binding sites in the L-type Ca2+ channel pore in rat phaeochromocytoma cells. J Physiol 466: 657–682, 1993
Jordan PC: How pore mouth charge distributions alter the permeability of transmembrane ionic channels. Biophys J 51: 297–311, 1987
Kuo C-C, Hess P: Ion permeation through the L-type Ca2+ chan nel in rat phaechromocytoma cells: Two sets of ion binding sites in the pore. J Physiol 466: 629–655, 1993
Patlack J, Horn R: Effect of N-bromoacetamide on single sodium channel currents in excized membrane patches. J Gen Physiol 79: 333–351, 1982
Aldrich RW, Corey DP, Stevens CF: A reinterpretation of mammalian sodium channel gating based on single channel recording. Nature 306: 436–441, 1983
Prod'hom B, Pietrobon D, Hess P: Interactions of protons with single open L-type calcium channels. Location of protonation site and dependence of proton-induced current fluctuations on concentration and species of permeation. J Gen Physiol 94: 23–42, 1989
Lipkind GM, Fozzard HA: A structural model of the tetrodotoxin and saxitoxin binding site of the Na+ channel. Biophys J 66: 1–13, 1994
Kuo C-C, Hess P: A functional view of the entrance of L-type Ca2+ channels: Estimates of the size and surface potential at the pore mouths. Neuron 9: 515–526, 1992
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bahinski, A., Yatani, A., Mikala, G. et al. Charged amino acids near the pore entrance influence ion-conduction of a human L-type cardiac calcium channel. Mol Cell Biochem 166, 125–134 (1997). https://doi.org/10.1023/A:1006847632410
Issue Date:
DOI: https://doi.org/10.1023/A:1006847632410