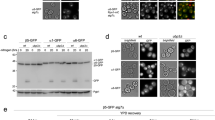
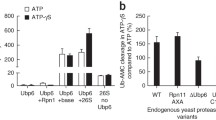
Abstract
The 26S proteasome is a 2-Megadalton proteolytic complex with over 30 distinct subunits. The 19S particle, a subcomplex of the 26S proteasome, is thought to confer ATP-dependence and ubiquitin-dependence on the proteolytic core particle of the proteasome. Given the complexity of the 19S particle, genetic approaches are likely to play an important role in its analysis. We have initiated biochemical and genetic studies of the 19S particle in Saccharomyces cerevisiae. Here we describe the localization to the proteasome of several ATPases that were previously proposed to be involved in transcription. Independent studies indicate that the mammalian 26S proteasome contains closely related ATPases. We have also found that the multiubiquitin chain binding protein Mcb1, a homolog of the mammalian S5a protein, is a subunit of the yeast proteasome. However, contrary to expectation, MCB1 is not an essential gene in yeast. The mcb1 mutant grows at a nearly wild-type rate, and the breakdown of most ubiquitin-protein conjugates is unaffected in this strain. One substrate, Ub-Proline-βgal, was found to require MCB1 for its breakdown, but it remains unclear whether Mcb1 serves as a ubiquitin receptor in this process. Our data suggest that the recognition of ubiquitin conjugates by the proteasome is a complex process which must involve proteins other than Mcb1.
Similar content being viewed by others
References
Hershko A, Ciechanover A, Heller H, Haas AL & Rose IA (1980) Proc. Nat. Acad. Sci. USA 77: 1783–1786
Finley D, Ciechanover A & Varshavsky A (1984) Cell 37: 43–55
Ciechanover A, Finley D & Varshavsky A (1984) Cell 37: 57–66
Rock KL, Gramm C, Rothstein L, Clark K, Stein R, Dick L, Hwang D & Goldberg A (1994) Cell 78: 761–771
Glotzer M, Murray AW & Kirschner MW (1991) Nature 349: 132–138
Yaglom J, Linskens MHK, Sadis S, Rubin DM, Futcher B & Finley D (1995) Mol. Cell Biol. 15: 731–741
Deshaies, RJ, Chau V & Kirschner MW (1995) EMBO J. 14: 303–312
Schwob E, Bohm T, Mendenhall MD & Nasmyth K (1994) Cell 79: 233–244
Pagano M, Tam SW, Theodoras AM, Beer—Romano P, Del Sal G, Chau V, Yew PR, Draetta GF & Rolfe M (1995) Science 269: 682–685
Maki cg, Huibregtse JM & Howley PM (1996) Cancer Res. 56: 2649–2654
Palombella V, Rando OJ, Goldberg AL & Maniatis T (1994) Cell 78: 773–785
Chen Z, Hagler J, Palombella VJ, Melandri F, Scherer D, Ballard D & Maniatis T (1995) Genes & Development 9: 1586–1597
Kim TK & Maniatis T (1996) Science 273: 1717–1719
Chen P, Johnson P, Sommer T, Jentsch S & Hochstrasser M (1993) Cell 74: 357–369
Kornitzer D, Raboy B, Kulka RG & Fink GR (1994) EMBO J. 13: 6021–6030
Rubin, DM & Finley D (1995) Curr. Biol. 3: 853–858
Coux O, Tanaka K & Goldberg AL (1996) Annu. Rev. Biochem. 65: 801–847
Löwe J, Stock J, Jap B, Zwickl P, Baumeister W & Huber R (1995) Science 268: 533–539
Wenzel T & Baumeister W (1995) Nature Struct. Biol. 2: 199–204
DeMartino GN, Moomaw CR, Zagnitko OP, Proske RJ, Chu—Ping M, Afendis SJ, Swaffield JC & Slaughter CA (1994) J. Biol. Chem. 269: 20 878–20 884
Hoffman L, Pratt G & Rechsteiner M (1992) J. Biol. Chem. 267: 22 362–22 368
Haracska L & Udvardy A (1995) Eur. J. Biochem. 231: 720–725
Peters J—M, Franke WW, Kleinschmidt JA (1994) J. Biol. Chem. 269: 7709–7718
Hartl FU (1996) Nature 381: 571–579
Dubiel W, Ferrel K, Pratt G & Rechsteiner M (1992) J. Biol. Chem. 267: 22 699–22 702
Rubin DM, Coux O, Wefes I, Hengartner C, Young R, Goldberg A & Finley D (1996) Nature 379: 655–657
Dubiel W, Ferrel K. & Rechsteiner M. (1995) Mol. Biol. Rep. 21: 27–34
Swaffield JC, Bromberg JF & Johnston SA (1992) Nature 357: 698–700
Swaffield JC, Melcher K & Johnston SA (1995) Nature 374: 88–91
Kim Y—J, Björklund S, Li Y, Sayre MH & Kornberg RD (1994) Cell 77: 599–608
Lee JW, Ryan F, Swaffield JC, Johnston SA & Moore DD (1995) Nature 374: 91–94
Shibuya H, Irie K, Ninomiya—Tsuji J, Goebl M, Taniguchi T & Matsumoto K (1992) Nature 357: 700–702
Nelbock P, Dillon PJ, Perkins A & Rosen CA (1990) Science 248: 1650–1653
Ohana B, Moore PA, Ruben SM, Southgate CD, Green MR & Rosen CA (1993) Proc. Nat. Acad. Sci. USA 90: 138–142
Ghislain M, Udvardy A & Mann C (1993) Nature 366: 358–362
Koleske AJ & Young RA (1995) Trends Biochem. Sci. 20: 113–116
Heinemeyer W, Kleinschmidt JA, Saidowsky J, Escher C & Wolf DH (1991) EMBO J. 10: 555–562
Akiyama K, Yakota K, Kagawa S, Shimbara N, DeMartino GN, Slaughter CA, Noda C & Tanaka K (1995) FEBS Lett. 363: 151–156
Deveraux Q, Ustrell V, Pickart C & Rechsteiner M (1994) J. Biol. Chem. 269: 7059–7061
van Nocker S, Deveraux Q, Rechsteiner M & Vierstra R (1996) Proc. Nat. Acad. Sci. USA 93: 856–860
van Nocker S, Sadis S, Rubin DM, Glickman M, Fu H, Coux O, Wefes I, Finley D & Vierstra RD (1996) Mol. Cell Biol. 16: 6020–6028
Ferrel K, Deveraux Q, van Nocker S & Rechsteiner M (1996) FEBS Lett. 381: 143–148
Deveraux Q, van Nocker S, Mahaffey D, Vierstra R & Rechsteiner M (1995) J. Biol. Chem. 270: 29 660–29 663
Finley D, Sadis S, Monia BP, Boucher P, Ecker DJ, Crooke ST & Chau V (1994) Mol. Cell Biol. 14: 5501–5509
Bachmair A, Finley D & Varshavsky A (1986) Science 234: 179–186
Jentsch S, McGrath JP & Varshavsky A (1987) Nature 329: 131–134
Johnson ES, Ma PCM, Ota IM & Varshavsky A (1995) J. Biol. Chem. 270: 17 442–17 456
Beal R, Deveraux Q, Xia G, Rechsteiner M, Pickart C (1996) Proc. Nat. Acad. Sci. USA 93: 861–866
Wilkinson KD, Tashayev VL, O'Conner LB, Larsen CN, Kasparek E & Pickart CM(1996) Biochemistry 34: 14 535–14 546
Reiss Y, Heller H & Hershko A (1989) J. Biol. Chem. 264: 10 378–10 383
Baboshina OV & Haas A (1996) J. Biol. Chem. 271: 2823–2831
Spence J, Sadis S, Haas AL & Finley D (1995) Mol. Cell Biol. 15: 1265–1273
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Rubin, D.M., van Nocker, S., Glickman, M. et al. ATPase and ubiquitin-binding proteins of the yeast proteasome. Mol Biol Rep 24, 17–26 (1997). https://doi.org/10.1023/A:1006844305067
Issue Date:
DOI: https://doi.org/10.1023/A:1006844305067