Abstract
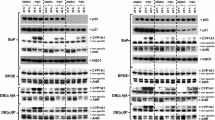
Exposure to ubiquitous environmental chemicals, such as polycyclic aromatic hydrocarbons (PAH), may contribute to human breast cancer. In animals, PAH induce tumors in part by activating the aryl hydrocarbon receptor (AhR)/transcription factor. Historically, investigations into AhR-regulated carcinogenesis have focused on AhR-dependent transcriptional regulation of cytochrome P450 (CYP) enzymes which oxidize PAH to mutagenic intermediates. However, recent studies suggest that the AhR directly regulates cell growth. Given the postulated role of the AhR in carcinogenesis, we predicted that: (1) tissue predisposed to PAH tumorigenesis would express the AhR and (2) aberrant AhR and/or AhR-regulated gene expression would accompany malignant transformation. To test these hypotheses, AhR and CYP1 protein and/or mRNA levels were evaluated in rat mammary tumors induced with 7, 12-dimethylbenz[a]anthracene (DMBA), a prototypic PAH and AhR ligand. Results indicate modest AhR expression in normal mammary myoepithelial and ductal epithelial cells. In contrast, high AhR levels were detected in DMBA-induced tumors. Nuclear AhR localization in tumors suggested constitutive AhR activation. In situ hybridization and quantitative RT-PCR assays indicated high AhR mRNA levels in neoplastic epithelial cells. While both AhR-regulated CYP1A1 and CYP1B1 mRNAs were induced in breast tissue within 6 h of DMBA gavage, only CYP1B1 mRNA remained elevated in tumors. These results: (1) help explain targeting of breast tissue by carcinogenic PAH, (2) imply that AhR and CYP1B1 hyper-expression represent molecular biomarkers for, at least, PAH-induced mammary cell transformation, and (3) suggest mechanisms through which the AhR may contribute to carcinogenesis well after exogenous AhR ligands have been eliminated.
Similar content being viewed by others
References
Hunter D, Hankinson S, Laden F, Colditz G, Manson J, Willett W, Speizer F, Wolff M: Plasma organochlorine levels and the risk of breast cancer. NE J Med 337: 1253–1258, 1997
Morris J, Seifter E: The role of aromatic hydrocarbons in the genesis of breast cancer. Medical Hypotheses 38: 177–184, 1993
Safe S: Xenoestrogens and breast cancer. NEJ Med 337: 1303–1305, 1997
Leung B, Sasaki G, Leung J: Estrogen-prolactin dependency in 7, 12-dimethyl-benz[a]anthracene-induced tumors. Cancer Res 35: 621–627, 1975
Medina D: Mammary tumorigenesis in chemical carcinogentreated mice. I. Incidence in Balb-c and C57BL/6 mice. J Natl Canc Inst 53: 213–221, 1974
Rogers A, Conner, B: Dimethylbenzanthracene-induced mammary tumorigenesis in ethanol-fed rats. Nutrition Res 10: 915–928, 1990
Russo J, Russo I, Zwieten MV, Rogers A, Gusterson B: Classification of neoplastic and non-neoplastic lesions of the rat mammary gland, Springer-Verlag, Berlin, 1989, p. 275–304
DiAugustine R, Davis D: A holistic approach to breast cancer. Environ Health Perspect 101: 116–120, 1993
Dusich K, Sigurson F, Hall W, Dean A: Cancer rates in a community exposed to low levels of creosote components in municipal water. Minn Med 63: 803–806, 1980
Falk F, Ricci J, Wolff M, Godbold J, Deckers P: Pesticides and polychlorinated biphenyl residues in human breast lipids and their relation to breast cancer. Arch Env Health 47: 143–149, 1992
Hoyer AP, Grandjean P, Jorgensen T, Brock JW, Hartvig HB: Organochlorine and breast cancer. Lancet 352: 1816–1820, 1998
Manz A, Berger J, Dwyer J, Flesch-Janys D, Nagel S, Waltsgott H: Cancer mortality among workers in chemical plant contaminated with dioxin. Lancet 338: 959–964, 1991
Shields P, Harris C: Molecular epidemiology and the genetics of environmental cancer. JAMA 266: 681, 1991
Wassermann M, Nogueira D, Tomatis L, Mirra A, Shibata H, Arie G, Cucos S, Wassermann D: Organochlorine compounds in neoplastic and adjacent apparently normal breast tissue. Bull Environ Contam and Toxicol 15: 479–483, 1976
Willett W: The search for the cause of breast and colon cancer. Nature 338: 389–394, 1989
Wolff M, Toniolo P, Lee E, Rivera M, Dubin N: Blood levels of organochlorine residues and risk of breast cancer. J Natl Canc Inst 85: 648–652, 1993
Lopez-Carrillo L, Blair A, Lopez-Cervantes M, Cebrian M, Rueda C, Reyes R, Mohar A, Bravo J: Dichlorodiphenyltrichloroethane serum levels and breast cancer risk: A casecontrol study from Mexico. Canc Res 57: 3728–3732, 1997
Dolwick K, Swanson H, Bradfield C: In vitro analysis of Ah receptor domains involved in ligand-activated DNA recognition. Proc Natl Acad Sci USA 90: 8566–8570, 1993
Okey A, Riddick D, Harper P: The Ah receptor: mediator of the toxicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) and related compounds. Toxicol Lett 70: 1–22, 1994
Schmidt J, Carver L, Bradfield C: Molecular characterization of the murine Ahr gene. J Biol Chem 268: 22203–22209, 1993
Carver LA, Bradfield CA: Ligand-dependent interaction of the aryl hydrocarbon receptor with a novel immunophilin homolog in vivo. J Biol Chem 272: 11452–11456, 1997
Chen HS, Perdew GH: Subunit composition of the heteromeric cytosolic aryl hydrocarbon receptor complex. J Biol Chem 269: 27554, 1994
Enan E, Matsumura F: Identification of c-Src as the integral component of the cytosolic Ah receptor complex, transducing the signal of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) through the protein phosphorylation pathway. Biochem Pharmacol 52: 1599–1612, 1996
Ma Q, Whitlock JJ: A novel cytoplasmic protein that interacts with the Ah receptor, contains tetratricopeptide repeat motifs, and augments the transcriptional respose to 2,3,7,8-tetrachlorodibenzo-p-dioxin. J Biol Chem 272: 8878–8884, 1997
Safe S, Krishan V: Cellular and molecular biology of aryl hydrocarbon (Ah) receptor-mediated gene expression. Arch of Toxicology 99–115, 1995
Gradin K, Wilhelmsson A, Poellinger L, Berghard A: Nonresponsiveness of normal human fibroblasts to dioxin correlates with the presence of a constitutive xenobiotic response element-binding factor. J Biol Chem 268: 4061–4068, 1993
Hoffer A, Chang C, Puga A: Dioxin induces trancription of fos and jun genes by Ah receptor-dependent and-independent pathways. Toxicol Appl Pharmacol 141: 238–247, 1996
Larsen M, Angus W, Brake P, Eltom S, Jefcoate C: CYP1B1 represents the major PAH-responsive P450 cytochrome constitutively expressed in normal primary HMEC. Fund Appl Toxicol 36: 24, 1997
Poellinger L, Gottlicher M, Gustafsson J: The dioxin and peroxisome proliferator-activated receptors: nuclear receptors in search of endogenous ligands. Trends Pharmacol Sci 13: 241–245, 1992
Puga A, Nebert D, Carrier F: Dioxin induces expression of c-fos and c-jun proto-oncogenes and a large increase in transcription factor AP-1. DNA and Cell Biol 11: 269–281, 1992
Smithe H, Lu Y, Deng G, Martinez O, Lrams S, Ljung B, Thor A, Lagios M: Molecular aspects of early stages of breast cancer progression. J Cell Biol 17G: 144–152, 1993
Kouri R, McKinney C, Slomiany D, Snodgrass D, Wray N, McLemore T: Positive correlation between high aryl hydrocarbon hydroxylase activity and primary lung cancer as analyzed in cryo preserved lymphocytes. Canc Res 42: 5030–5037, 1982
Nebert D: Proposed role of drug-metabolizing enzymes: regulation of steady state levels of the ligand that effect growth, homeostasis, thyroid hormone. Nature 324: 635–640, 1987
Rigdon R, Neal J: Relationship of leukemia to lung and stomach tumors in mice fed benzo[a]pyrene. Proc Soc Exptl Biol 130: 146–148, 1969
Nebert D, Petersen D, Fornace AJ: Cellular responses to oxidative stres: The [Ah] gene battery as a paradigm. Environ Health Persp 88: 13–25, 1990
Vaziri C, Faller D: A benzo[a]pyrene-induced cell cycle checkpoint resulting in p53-independent G1 arrest in 3T3 fibroblasts. J Biol Chem 272: 2762–2769, 1997
Vaziri C, Schneider A, Sherr D, Faller D: Expression of the aryl hydrocarbon receptor is regulated by serum and mitogenic growth factors in murine 3T3 fibroblasts. J Biol Chem 271: 25921–25927, 1996
Bombick D, Jankun J, Tullis K, Matsumura F: 2,3,7,8-Tetrachlorodibenzo-p-dioxin causes increases in expression of c-erb-A and levles of protein-tyrosine kinases in selected tissues of responsive mouse strains. Proc Natl Acad Sci USA 85: 4128, 1988
Sadhu D, Merchant M, Safe S, Ramos K: Modulation of protooncogene expression in rat aortic smoothe muscle cell by benzo[a]pyrene. Arch Biochem and Biophysics 300: 124–131, 1993
Ma X, Babish J: Acute 2,3,7,8-tetrachlorodibenzo-p-dioxin exposure results in enhanced tyrosylphosphorylation and expression of murine hepatic cyclin dependent kinases. Biochem Biophys Res Comm 197: 1070–1077, 1993
Ma X, Wheelock G, Dong H, Babish J: Treatment of guinea pig hepatic cytosol with TCDD results in Ah receptor complexing with mitogen-activated protein kinase and rearranging cell cycle subunits. Fund Appl Toxicol 36: 128, 1997
Ge N-L, Elferink CJ: A direct interaction between the aryl hydrocarbon receptor and retinoblastoma protein. J Biol Chem 273: 22708–22713, 1998
Hushka DR, Hushka LJ, Williams JS, Greenlee WF: Aryl hydrocarbon receptor-dependent control of Rb phosphorylaion and G1/S progression in rat hepatoma cells. Proc Am Assoc Canc Res 39: 252, 1998
Kim DW, Gazourian L, Quadri SA, Sherr DH, Soneshein GE: The aryl hydrocarbon receptor/transcription factor (AhR) and the Rel A nuclear factor-κB subunit cooperate to transactivate the c-myc promoter. Submitted for publication, 2000
Tian Y, Ke S, Denison MS, Rabson AB, Gallo MA: Ah receptor and NF-κB interactions, a potential mechanism for dioxin toxicity. J Biol Chem 274: 510–515, 1999
Wotiz H, Muller DBR: Effects of estrogen on DMBA-induced breast tumors. J Steroid Biochem 20: 1067–1075, 1984
Russo J, Gusterson BA, Rogers AE, Russo IH, Wellings SR, Awieten MJV: Biology of disease: Compartive study of human and rat mammary tumorigenesis. Lab Invest 62: 244–278, 1990
Yamaguchi K, Near RI, Matulka RA, Shneider A, Toselli P, Trombino AF, Sherr DH: Activation of the aryl hydrocarbon receptor/transcription factor and stromal cell dependent preB cell apoptosis. J Immunol 158: 2165–2173, 1997
Siebert P, Larrick J: PCR MIMICS: Competitive DNA fragments for use as internal standards in quantitative PCR. Biotechniques 14: 244–249, 1993
Singh S, Perdew G: Alterations in the AhR level after staurosporine treatment. Arch Biochem Biophys 305: 170, 1993
Rubin E, Farber J: Pathology, Second edn, J.B. Lippincott Company, Philadelphia 1994, p. 959
Jue SF, Bradley RS, Rudnicki JA, Varmus HE, Brown AM: The mouse Wnt-1 gene can act via a paracrine mechanism in transformation of mammary epithelial cells. Mol Cell Biol 12: 321–328, 1992
Noel A, Foidart J-M: The role of stroma in breast carcinoma growth in vivo. J Mamm Gland Biol Neoplasia 3: 215–225, 1998
Woodward TL, Xie JW, Haslam SZ: The role of mammary stroma in modulating the proliferative response to ovarian hormones in the normal mammary gland. J Mamm Gland Biol Neoplasia 3: 117–213, 1998
Cuthill S, Poellinger L, Gustafsson J-A: The receptor for 2,3,7,8-tetrachlorodibenzo-p-dioxin in the mouse hepatoma cell line Hepa 1c1c7. J Biol Chem 262: 3477–3481, 1987
Numayama-Tsuruta K, Kobayashi A, Sogawa K, Fujii-Kuriyama Y: A point mutation responsible for defective function of the aryl-hydrocarbon-receptor nuclear translocator in mutant Hepa-1c1c7 cells. Eur J Biochem: 246: 486–495, 1997
Davarinos NA, Pollenz RS: Aryl hydrocarbon receptor imported into the nucleus following ligand binding is rapidly degraded via the cytoplasmic proteosome following nuclear export. J Biol Chem 274: 28708–28715, 1999
Giannone JV, Li W, Markus P, Okey AB: Prolonged depletion of AH receptor without alteration of receptor mRNA levels after treatment of cells in culture with 2,3,7,8-tetrachlorodibenzo-p-dioxin. Biochem Pharm 55: 489–497, 1998
Arellano L, Wang X, Safe S: Effects of cyloheximide on the induction of CYP1A1 gene expression by 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in three human breast cancer cell lines. Carcinogenesis 14, 219, 1993
Dong L, Ma Q, Whitlock JJ: DNA binding by the heterodimeric Ah receptor. Relationship to dioxin-induced CYP1A1 transcription in vivo. J Biol Chem 271: 7942–7948, 1996
Elangovan V, Sekar N, Govindasamy S: Chemopreventive potential of dietary bioflavonoids against 20-methylcholanthrene-induced tumorigenesis. Canc Lett 87: 107–113, 1994
Eltom SE, Larsen MC, Jefcoate CR: Expression of CYP1B1 but not CYP1A1 by primary cultured human mammary stromal fibroblasts constitutively and in response to dioxin exposure: Role of the Ah receptor. Carcinogenesis 19: 1437–1444, 1998
Forkert P: Coexpression of Ah receptor and CYP1A1 in hepatocytes of C57BL/6J and DBA/2J mice. Toxicol Appl Pharmacol 142: 69–78, 1997
Lu Y, Santostefano M, Cunningham B, Threadgill M, Safe S: Identification of 30-methoxy-40-nitroflavone as a pure aryl hydrocarbon (Ah) receptor antagonist and evidence for more than one form of the nuclear Ah receptor in MCF-7 human breast cancer cells. Arch Biochem Biophys 316: 470–477, 1995
Nebert D, Petersen D, Puga A: Human AH locus polymorphism and cancer: inducibility of CYP1A1 and other genes by combustion products and dioxin. Pharmacogenetics 1: 68–78, 1991
Spink D, Spink B, Cao J, DePasquale J, Pentecost B, Li Y, Sutter T: Differential induction of CYP1A1 and CYP1B1 by TCDD in human breast epithelial cells and breast tumor cells. Fund Appl Toxicol 36: 24, 1997
Taioli E, Trachman J, Chen X, Toniolo P, Garte S: A CYP1A1 restriction fragment length polymorphism is associated with breast cancer in African–American women. Canc Res 55: 3757–3758, 1995
Walker N, Lax S, Wyde M, Crofts F, Lucier C, Sutter T: CYP1B1 and CYP1A1 exhibit differential inducibility by TCDD in a Sprague-Dawley rat tumor promotion model. Fund Appl Toxicol 36: 215, 1997
Schmidt J, Su G, Simon JRM, Bradfield C: Characterization of a murine Ahr null allele: Involvement of the Ah receptor in hepatic growth and development. Proc Natl Acad Sci USA 93: 6731–6736, 1996
Zhang L, Savas U, Alexander DL, Jefcoate CR: Characterization of the mouse Cyp1B1 gene. Identification of an enhancer region that directs aryl hydrocarbon receptor-mediated constitutive and induced expression. J Biol Chem 273: 5174–5183, 1998
Christou M, Savas U, Schroeder S, Shen X, Thompson T, GouldMN, Jefcoate CR: Cytochromes CYP1A1 and CYP1B1 in the rat mammary gland: cell-specific expression and regulation by polycyclic aromatic hydrocarbons and hormones. Mol Cell Endocrinol 115: 41–51, 1995
Kress S, Greenlee WF: Cell-specific regulation of human CYP1A1 and CYP1B1 genes. Canc Res 57: 1264–1269, 1997
Murray GI, Taylor MC, McFadyen MCE, McKay JA, Greelee WF, Burke MD, Melvin WT: Tumor-specific expression of cytochrome P450 CYP1B1. Canc Res 57: 3026–3031, 1997
Spink DC, Spink BC, Cao JQ, DePasquale JA, Pentecost BT, Fasco MJ, Li Y, Sutter TR: Differential expression of CYP1A1 and CYP1B1 in human breast epithelial cells and breast tumor cells. Carcinogenesis 19: 291–298, 1998
Christou M, Moore C, Gould M, Jefcoate C: Induction of mammary cytochromes P-450: an essential first step in the metabolism of 7,12-dimethylbenz[a]anthracene by rat mammary epithelial cells. Carcinogenesis 8: 73–80, 1987
Thomsen J, Wang X, Safe S: Induction of CYP1A1 gene expression in human breast cancer cells: A role for the estrogen receptor, Society of Toxicology Annual Meeting, pp. 237, 1995
Sovak MA, Bellas RE, Kim DW, Zanieski GJ, Rogers AE, Traish AM, Sonenshein GE: Aberrant nuclear factor-κB/Rel expression and the pathogenesis of breast cancer. J Clin Invest 100: 2952–2960, 1997
Sonenshein GE: Rel/NF-kappa B transcription factors and the control of apoptosis. Seminars in Cancer Biology 8: 113–119, 1997
Pollenz RS, Santostefano MJ, Klett E, Richardson VM, Birnbaoum LS: Female Sprague-Dawley rats exposed to a single oral dose of 2,3,7,8-tetrachlorodibenzo-p-dioxin exhibit sustained depletion of aryl hydrocarbon receptor protein in liver, spleen, thymus and lung. Toxicol Sci 42: 117–128, 1998
Gammal EB, Carrol KK, Plunkett ER: Effects of dietary fat on the uptake and clreance of 7,12-dimethylbenz[alpha]-anthracene by rat mammary tissue. Canc Res 28: 384–385, 1968
Chang C, Puga A: Constitutive activation of the aromatic hydrocarbon receptor. Mol Cell Biol 18: 525–535, 1998
Shigh SS, Hord NG, Perdew GH: Characterization of the activated form of the aryl hydrocarbon receptor in the nucleus of HeLa cells in the absence of exogenous ligand. Arch Biochem Biophys 329: 47–55, 1996
Wang X, Thomsen JS, Santostefano M, Rosengren R, Safe S, Perdew GH: Comparative properties of the nuclear aryl hydrocarbon (Ah) receptor complex from several human cell lines. European J Pharmacol 293: 191–205, 1995
Ma Q, Jr JW: The aromatic hydrocarbon receptor modulates the Hepa 1c1c7 cell cycle and differentiated state independently of dioxin. Mol Cell Biol 16: 2144–2150, 1996
Dohr O, Abel J: Transforming growth factor-β1 co-regulates mRNA expression of aryl hydrocarbon receptor and cell cycleregulating genes in human cancer cell lines. Biochem Biophys Res Commun 8: 86–91, 1997
Cover CM, Hsieh SJ, Tran SH, Hallden G, Kim GS, Bjeldanes LF, Firestone GL: Indole-3-carbinol inhibits the expression of cyclin-dependent kinase-6 and induces a G1 cell cycle arrest of human breast cancer cells independent of estrogen receptor signaling. J Biol Chem 273: 3838–3847, 1998
McDougal A, Wilson C, Safe S: Inhibition of 7,12-dimethylbenz[ a]anthracene-induced rat mammary tumor growth by aryl hydrocarbon receptor agonist. Canc Lett 120: 53–63, 1997
Merchant M, Krishan V, Safe S: Mechanism of action of a-naphthoflavone as an Ah receptor antagonist in MCF-7 human breast cancer cells. Toxicol Appl Pharmacol 120: 179, 1993
Chaloupka K, Krishan V, Safe S: polynuclear aromatic hydrocarbon carcinogens as antiestrogens in MCF-7 human breast cancer cells: role of the Ah receptor. Carcinogen 13: 2233, 1992
Harris M, Zacharewski, Safe S: Effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin and related compounds on the occupied nuclear estrogen receptor in MCF-7 human breast cancer cells. Canc Res 50: 3579–3584, 1990
Safe S: Modulation of gene expression and endocrine response pathways by 2,3,7,8-tetrachlorodibenzo-p-dioxin and related compounds. Pharmacol Ther 67: 247–281, 1995
McKay J, Melvin W, Ah-See A, Ewen S, Greenlee W, Marcus C, Burke M, Murray G: Expression of cytochrome P450 CYP1B1 in breast cancer. FEBS Letters 374: 270–272, 1995
Shimada T, Hayes CL, Yamazaki H, Amin S, Hecht SS, Guengerich FP, Sutter TR: Activation of chemically diverse procarcinogens by human cytochrome P450 1B1. Canc Res 56: 2979–2984, 1996
Liehr JG, Ricci MJ: 4-Hydroxylation of estrogens as a marker of human mammary tumors. Proc Natl Acad Sci USA 93: 3294–3296, 1996
Liehr JG, Ricci MJ, Jefcoate CR, Hannigan EV, Hkanson JA, Zhu BT: 4-Hydroxylation of estradiol by human uterine myometrium and myoma microsomes: Implication for the mechanism of uterine tumorigenesis. Proc Natl Acad Sci USA 92: 9220–9224, 1995
Rights and permissions
About this article
Cite this article
Trombino, A.F., Near, R.I., Matulka, R.A. et al. Expression of the aryl hydrocarbon receptor/transcription factor (AhR) and AhR-regulated CYP1. Breast Cancer Res Treat 63, 117–131 (2000). https://doi.org/10.1023/A:1006443104670
Issue Date:
DOI: https://doi.org/10.1023/A:1006443104670