Abstract
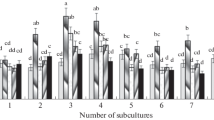
The involvement of ethylene in the vitro development of shoots from nodal segments of two cultivars of carnation (Dianthus caryophyllus L.) was studied. Shoots of cv. Barbaret Antares showed low hyperhydricity in contrast with the high levels showed by cv. Barbaret Tanga when both were cultured in airtight culture vessels. Longer shoots were produced, in both cases, when the rate of gas exchange in the culture vessel was increased by using vented closures, which also prevented hyperhydricity and increased the multiplication coefficient in cultures of Barbaret Tanga.
The two cultivars produced ethylene throughout the culture period but, a higher amount was produced during the first, second and fourth weeks in culture by the cultivar more sensitive to ventilation (Barbaret Tanga). Trapping ethylene did not produce any effect on cv. Barbaret Antares but improved the quality of cv. Barbaret Tanga explants, decreasing hyperhydricity and increasing the number of shoots, the length of the main shoot and the multiplication coefficient. These effects were more marked when ethylene was trapped during the first two weeks in culture.
Similar content being viewed by others
References
Abeles FB, Morgan PW and Saltveit ME Jr. (1992) Ethylene in Plant Biology (2nd edn). New York: Academic Press Inc
De Proft MP, Maene LJ and Debergh PC (1985) Carbon dioxide and ethylene evolution in the culture atmosphere of Magnolia cultured in vitro. Physiol Plant 65: 375–379
Debergh PC, Aitken-Christie J, Cohen D, Grout B, Von Arnold S, Zimmerman R and Ziv M (1992) Reconsideration of the term “vitrification” as used in micropropagation. Plant Cell Tissue Organ Cult 30: 135–140
Demeester JJ, Matthijs DG, Pascat B and Debergh PC (1995) Toward a controllable headspace composition. Growth, development, and headspace of a micropropagated Prunus rootstock in different containers. In Vitro Cell Dev Biol 31P: 105–112
Dillen W and Buysens S (1989) A simple technique to overcome vitrification in Gypsophila paniculata L. Plant Cell Tissue Organ Cult 19: 181–188
Dimasi-Theriou K, Economou AE and Sfakiotakis EM (1993) Promotion of petunia (Petunia hybrida L.) regeneration in vitro by ethylene. Plant Cell Tissue Organ Cult 32: 219–225
Dolev E (1986) Hardening of tissue cultured fern and carnation plantlets. MSc Thesis. Israel: The Hebrew University of Jerusalem
Fujiwara K and Kozai T (1995) Physical microenvironment and its effects. In: Aitken-Christie J, Kozai T and Smith ML (eds) Automation and Environmental Control in Plant Tissue Culture, pp 319–336. Dordrecht: Kluwer Academic Publisher
González A, Arigita L, Majada JP and Sánchez Tamés R (1997) In vitro organogenesis and plant growth of Populus tremula in relation to ethylene. Plant Growth Regul 22: 1–6
Kevers C and Gaspar T (1985) Vitrification of carnation in vitro: Changes in ethylene production, ACC level and capacity to convert ACC to ethylene. Plant Cell Tissue Organ Cult 4: 215–223
Kumar PP, Reid DM and Thorpe TA (1987) The role of ethylene and carbon dioxide in differentiation on shoot buds in excised cotyledons of Pinus radiata in vitro. Physiol Plant 69: 244–252
Lambardi M, Benelli C and Fabbri A (1997) In vitro axillary shoot proliferation of apple rootstocks under different ethylene conditions. In Vitro Cell Dev Biol 33P: 70–74
Majada JP, Fal MA and Sánchez-Tamés R (1997) The effect of ventilation rate on proliferation and hyperhydricity of Dianthus caryophyllus L. In Vitro Cell Dev Biol 33P: 62–69
McClelland MT and Smith MAL (1990) Vessel type, closure, and explant orientation influence in vitro performance of five woody species. HortScience 25: 797–800
Melé E, Messeguer J and Camprubi P (1982) Effects of ethylene on carnation explants grown in sealed vessels. In: A Fujiwara (ed) Plant Tissue Culture 1982. Proc. IAPTC, pp 69–70. Tokyo
Monette PL (1983) Influence of size of culture vessel on in vitro proliferation of grape in a liquid medium. Plant Cell Tissue Organ Cult 2: 327–332
Murashige T and Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol Plant 15: 473–497
Righetti B and Facini O (1992) Headspace gas composition in four Prunus avium cultivars with differing photosynthetic capabilities. In Vitro Cell Dev Biol 28P: 179–182
Righetti B, Magnanini E, Infante R and Predieri S (1990) Ethylene, ethanol, acetaldehyde and carbon dioxide released by Prunus avium shoot cultures. Physiol Plant 78: 507–510
Rossetto M, Dixon KW and Bunn E (1992) Aeration: A simple method to control vitrification and improve in vitro culture of rare australian plants. In Vitro Cell Dev Biol 28P: 192–196
Sisler EC and Wood C (1988) Interaction of ethylene and CO2. Physiol Plant 73: 440–444
Smith MAL and Spomer LA (1995) Vessels, gels, liquid media, and support systems. In: Aitken-Christie J, Kozai T and Smith ML (eds) Automation and Environmental Control in Plant Tissue Culture, pp 371–404. Dordrecht: Kluwer Academic Publisher
Ward TM, Wright M, Roberts JA, Self R and Osborne DJ (1978) Analytical procedures for the assay and identification of ethylene. In: Hillman JR (ed) Isolation of Plant Growth Substances, pp 135–151. London: Cambridge University Press
Ziv M (1991) Vitrification: morphological and physiological disorders of in vitro plants. In: Debergh PC and Zimmerman RH (eds) Micropropagation, pp 45–70. Dordrecht: Kluwer Academic Publisher
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Fal, M., Majada, J., González, A. et al. Differences between Dianthus caryophyllus L. cultivar in in vitro growth and morphogenesis are related to their ethylene production. Plant Growth Regulation 27, 131–136 (1999). https://doi.org/10.1023/A:1006107230678
Issue Date:
DOI: https://doi.org/10.1023/A:1006107230678