Abstract
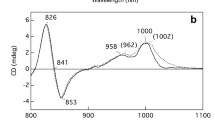
Low temperature absorption and linear dichroism (LD) measurements were performed on oriented membranes containing wild type Rhodobacter sphaeroides reaction centers, a mutant reaction center with the change Phe M197 to Arg (FM197R), and a double mutant reaction center where, in addition, Gly M203 was replaced by Asp (FM197R/GM203D). The monomeric bacteriochlorophyll band (B), which is highly congested in the wild type reaction center, was separated into two bands in the mutant reaction centers peaking 10 nm (single mutant) or 15 nm (double mutant) apart. This separation arose principally from changes in the interaction of the protein with the L-side monomer bacteriochlorophyll BL.The ability to separate the B bands is extremely useful in spectroscopic studies. The orientations of the two monomer-type transitions contributing to the B band were similar in all three reaction centres studied, and were asymmetric with respect to the orientation axis, with the transition mostly associated with BL making a smaller angle with the C2 axis. Differences in the LD observed in wild type membrane-bound or isolated reaction centers can be ascribed either to differences in shifts of the B transitions or to differences in the orientation axis.
Similar content being viewed by others
References
Beekman LMP, van Stokkum IHM, Monshouwer R, Rijnders AJ, McGlynn P, Visschers RW, Jones MR and van Grondelle R (1996) Primary electron transfer in membrane-bound reaction centers with mutations at the M210 position. J Phys Chem 100: 7256–7268
Breton J (1985) Orientation of chromophores in the reaction center of Rhodopseudomonas viridis. Comparison of low-temperature linear dichroism spectra with a model derived from X-ray crystallography. Biochim Biophys Acta 810: 235–245
Breton J (1988) Low temperature linear dichroism study of the orientation of the pigments in reduced and oxidized reaction centers of Rps. viridis and Rb. sphaeroides. In Breton J and Verméglio A (eds) The Photosynthetic Bacterial Reaction Center, Structure and Dynamics, pp 59–69. Plenum, New York
Breton J, Bylina EJ and Youvan DC (1989) Pigment organization in geneticaly modified reaction centers of Rhodobacter capsulatus. Biochemistry 26: 6423–6430
Breton J, Martin JL, Lambry JC, Robles SJ and Youvan DC (1990) Ground state and femtosecond transient absorption spectroscopy of a mutant of Rhodobacter capsulatus which lacks the initial electron acceptor bacteriopheophytin. In: Michel-Beyerle ME (ed) Reaction Centers of Photosynthetic Bacteria, pp 293–302. Springer, Berlin
Deisenhofer J and Michel H (1989) The photosynthetic reacion center from the purple bacterium Rhodopseudomonas viridis. Science 245: 1463–1473
Ermler U, Fritzsch G, Buchanan SK and Michel H (1994) Structure of the photosynthetic reaction centre from Rhodobacter sphaeroides at 2.65 Å resolution: Cofactors and protein-cofactor interactions. Structure 2: 925–936
Feher G, Allen JP, Okamura MY and Rees DC (1989) Structure and function of bacterial photosynthetic reaction centers. Nature 339: 111–116
Hanson LK (1988) Theoretical calculations of photosynthetic pigments. Photochem Photobiol 47: 903–921
Jones MR, Visschers RW, van Grondelle R and Hunter CN (1992) Construction and characterization of a mutant of Rhodobacter sphaeroides with the reaction center as the sole pigment–protein complex. Biochemistry 31: 4458–4465
Jones MR, Heer-Dawson M, Mattioli TA, Hunter, CN and Robert B (1994) Site-specific mutagenesis of the reaction centre from Rhodobacter sphaeroides studied by Fourier-transform Raman spectroscopy: Mutations at tyrosine M210 do not affect the electronic structure of the primary donor. FEBS Lett 339: 18–24
Kirmaier C and Holten D (1987) Primary photochemistry of reaction centers from the photosynthetic purple bacteria. Photosynth Res 13: 225–260
Mattioli TA, Lin X, Allen JP and Williams JC (1995) Correlation between multiple hydrogen bonding and alteration of the oxidation potential of the bacteriochlorophyll dimer of reaction centers from Rhodobacter sphaeroides. Biochemistry 34: 6142–6152
McAuley-Hecht KE, Fyfe PK, Ridge JP, Prince SM, Hunter CN, Isaacs NW, Cogdell RJ and Jones MR (1998) Structural studies of wild type and mutant reaction centres from an antennadeficient strain of Rhodobacter sphaeroides: Monitoring the optical properties of the complex from cell to crystal. Biochemistry (in press)
Scherer POJ and Fischer SF (1991) Interpretation of optical reaction center spectra. in Scheer H (ed) Chlorophylls, pp 1079–1093. CRC, Boca Raton, FL
Vos MH, Lambry JC, Robles SJ, Youvan DG, Breton J and Martin JL (1992) Femtosecond spectral evolution of the excited state of bacterial reaction centers at 10 K. Proc Natl Acad Sci USA. 88: 8885–8889
Vos MH, Jones, MR, McGlynn P, Hunter CN, Breton J. and Martin JL (1994) Influence of the membrane environment on vibrational motions in reaction centers of Rhodobacter sphaeroides. Biochim Biophys Acta 1186: 117–122
Vos MH, Jones MR, Breton J, Lambry JC and Martin JL (1996) Vibrational dephasing in long-and short-lived primary donor excited states in mutant reaction centers of Rhodobacter sphaeroides. Biochemistry 35: 2687–2692
Vos MH, Breton J and Martin JL (1997) Electronic energy transfer within the hexamer cofactor system of bacterial reaction centers. J Phys Chem: 101: 9820–9832
Williams JC, Alden RG, Murchison HA, Peloquin JM, Woodbury NW and Allen JP (1992) Effects of mutations near the bacteriochlorophylls in reaction centers from Rhodobacter sphaeroides. Biochemistry 31: 11029–11037
Woodbury NW, Peloquin JM, Alden RG, Lin X, Lin S, Taguchi AKW, Williams JC and Allen JP (1994) Relationship between thermodynamics and mechanism during photoinduced charge separation in reaction centers from Rhodobacter sphaeroides. Biochemistry 33: 8101–8112
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Vos, M.H., Rischel, C., Breton, J. et al. Linear dichroism of membrane-bound reaction centers from Rhodobacter sphaeroides: Alterations of the B band induced by site-specific mutations. Photosynthesis Research 55, 181–187 (1998). https://doi.org/10.1023/A:1005951717594
Issue Date:
DOI: https://doi.org/10.1023/A:1005951717594