Abstract
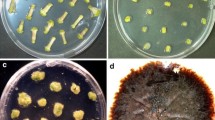
Young inflorescences and long-term culture calluses of wheat (Triticum aestivum L.) were used to investigate the effect of low temperature treatment on the potential of plant regeneration. The results indicated that the frequency of callus induction from immature inflorescences was decreased when treated for a long time at 5 °C. However, it was found that a 5 °C treatment significantly improved the regeneration frequency of calluses. The clear difference of peroxidases of wheat calluses was apparent after the cultures were treated at the low temperature. The isozyme band 8 became clearly faint, however, bands 6 and 9 became intense.
Similar content being viewed by others
References
Asano Y, Ito Y, Ohara M, Sugiura K & Fujiie A (1994) Improved regeneration response of creeping bentgrass and japonica rice by maltose and lactose. Plant Cell Tiss. Org. Cult. 39: 101–103
Bercetche J, Galerne M & Dereuddre J (1990) Efficient regeneration of plantlets from embryogenic callus of Picea abies (L.) Karst after freezing in liquid nitrogen. C. R. Acad. Sci. Paris (III): 357–363
Chaturvedi HC & Mitra GC (1975) A shift in morphogenetic pattern in Citrus callus tissue during prolonged culture. Ann. Bot. 39: 683–687
Chaturvedi HC & Jain M (1994) Restoration of regeneration potentiality in prolonged culture of Digitalis purpurea. Plant Cell Tiss. Org. Cult. 38: 73–75
Cocking EC, Davey MR, Pental D & Power JB (1981) Aspects of plant genetic manipulation. Nature. 293: 265–270
Dale PJ & Webb KJ (1985) Germplasm storage and micropropagation. In: Bright SWJ & Jones MGK (eds) Cereal Tissue and Cell Culture. (pp 76–96). Martinus Nijhoff/Dr W. Junk publishers, Lancaster
Datta SK, Datta K & Potrykus I (1990) Embryogenesis and plant regeneration from microspores of both ‘indica’ and ‘japonica’ rice. Plant Sci. 67: 83–88
Davis BJ (1964) Disc electrophoresis. Method and application to human serum proteins. Ann. N. Y. Acad. Sci. 121: 404–427
Joersbo M, Anderson JM, Okkels FT & Rajagopal R (1989) Isoperoxidases as markers of somatic embryogenesis in carrot cell suspension cultures. Physiol. Plant. 76: 10–16
Larkin PJ & Scowcroft WR (1981) Somaclonal variation-a novel source of variability from cell cultures for plant improvement. Theor. Appl. Genet. 60: 197–214
Lin H & Cheng JC (1990) Temperature influences to the somatic embryo development of two wildlife gramineous plants. Plant Physiol. Commun. (in Chinese) 4: 80
Liu EH (1973) A simple method of determining the relative actives of individual peroxidase isoenzymes in a tissue extract. Ann. Biochem. 56: 149–154
Martensson B & Widell S (1993) Pollen from cold-treated Nicotiana tabacum buds: Embryogenic capacity, peroxidase activity and partitioning in aqueous two-phase systems. Plant Cell Tiss. Org. Cult 35: 141–149
Murashige T & Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco cultures. Physiol. Plant. 15: 473–497
Nabors MW, Heyser JW, Dykes TA & Demott KJ (1983) Longduration, high-frequency plant regeneration from cereal tissue culture. Planta. 157: 385–391
Orshinsky BR & Tomes DT (1985) Effect of long term culture and low-temperature incubation on plant regeneration from a callus line of Birdsfoot trefoil. J. Plant Physiol. 119: 389–397
Powell W (1988) The influence of genotype and temperature pretreatment on anther culture response in barley. Plant Cell Tiss. Org. Cult. 12: 291–297
Rao KV, Suprasanna P & Reddy GM (1990) Biochemical changes in embryogenic and nonembryogenic call of Zea mays L. Plant Sci. 66: 127–130
Vain P, Yean H & Flament P (1989) Enhancement of production and regeneration of embryogenic type callus in Zea mays L. by AgNO3. Plant Cell Tiss. Org. Cult. 18: 143–151
Xu ZY, Pan GZ, He MY & Wang TY (1992) Application of low temperature storage to the preservation of embryogenic capacity in tobacco and carrot. Plant Physiol. Commun. (in Chinese) 28: 407–411
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hou, B., Yu, H. & Teng, S. Effects of low temperature on induction and differentiation of wheat calluses. Plant Cell, Tissue and Organ Culture 49, 35–38 (1997). https://doi.org/10.1023/A:1005854301398
Issue Date:
DOI: https://doi.org/10.1023/A:1005854301398