Abstract
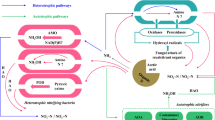
Ammonia conversion processes are essential for most soil and aquatic systems. Under natural conditions, the many possible reactions are difficult to analyze. For example, nitrification and denitrification have long been regarded as separate phenomena performed by different groups of bacteria in segregated areas of soils, sediments or aquatic systems sequentially in time. It has now been established that strict segregation in place and time of the two processes is not necessary and that both denitrifiers and nitrifiers have versatile metabolisms. However, the rates described for aerobic denitrifiers are very low compared to the rates observed under anoxic conditions. Also the rates of nitrifier denitrification are quite low, indicating that these conversions may not play an important role under natural conditions. In addition, these processes often result in the emission of quite large amounts of undesirable products, NO and N2O. Heterotrophic nitrification might be of relevance for systems, that contain a high carbon to nitrogen ratio. Recently, a novel process (Anammox) has been discovered in which ammonium serves as the electron donor for denitrification of nitrite into dinitrogen gas. 15N labeling studies showed that hydrazine and hydroxylamine were important intermediates in this process. Enrichment cultures on ammonium, nitrite and bicarbonate resulted in the dominance of one morphotypical microorganism. The growth rate of the cultures is extremely low (doubling time 11 days), but the affinity for ammonium and nitrite and the conversion rates (9.2 10−4 mol kg−1 s−1) are quite high. Some of the reported high nitrogen losses in soil and aquatic systems might be attributed to anaerobic ammonium oxidation. In addition, this conversion offers new opportunities for nitrogen removal, when it is combined with recently developed processes for partial nitrification.
Similar content being viewed by others
References
Abeliovich A 1987 Nitrifying bacteria in wastewater reservoirs. Appl. Environ. Microbiol. 53, 754–760.
Abeliovich A and Vonshak A 1992 Anaerobic metabolism of Nitrosomonas europaea. Arch. Microbiol. 158, 267–270.
Baumann B, Snozzi M, Zehnder A J B and Vandermeer J R 1996 Dynamics of denitrification activity of Paracoccus denitrificans in continuous culture during aerobic–anaerobic changes. J. Bacteriol. 178, 4367–4374.
Baumann B, Snozzi M, Zehnder A J B and Vandermeer J R 1997a Development of stable denitrifying cultures during repeated aerobic–anaerobic transient periods. Wat. Res. 31, 1947–1954.
Baumann B, Vandermeer J R, Snozzi M and Zehnder A J B 1997b Inhibition of denitrification activity but not of mRNA induction in Paracoccus denitrificans by nitrite at a suboptimal pH. Antonie van Leeuwenhoek 72, 183–189.
Bell L C, Richardson D J and Ferguson S J 1990 Periplasmic and membrane-bound respiratory nitrate reductases in Thiosphaera pantotropha. The periplasmic enzyme catalyzes the first step in aerobic denitrification. FEBS Lett. 265, 85–87.
Berks B C, Ferguson S J, Moir JWR and Richardson D J 1995a Enzymes and associated electron transport systems that catalyse the respiratory reduction of nitrogen oxides and oxyanions. Biochim. Biophys. Acta 1232, 97–173
Berks B C, Richardson D J, Reilly A, Willis A C and Ferguson S J 1995b The napEDABC gene cluster encoding the periplasmic nitrate reductase system of Thiosphaera pantotropha. Biochem. J. 309, 983–992.
Bock E, Schmidt I, Stuven R and Zart D 1995 Nitrogen loss caused by denitrifying Nitrosomonas cells using ammonium or hydrogen as electron donors and nitrite as electron acceptor. Arch. Microbiol. 163, 16–20.
Bodelier P L E, Libochant J A, Blom C W P M and Laanbroek H J 1998 Dynamics of nitrification and denitrification in root oxygenated sediments and adaptation of ammonia-oxidizing bacteria to low-oxygen or anoxic habitats. Appl. Environ. Microbiol. 62, 4100–4107.
Burrell P C, Keller J and Blackall L L 1998 Microbiology of a nitrite-oxidizing bioreactor. Appl. Environ. Microbiol. 64, 1878–1883.
Carter J P, Hsiao Y S Spiro S and Richardson D J 1995a Soil and sediment bacteria capable of aerobic nitrate respiration. Appl. Environ. Microbiol. 61, 2852–2858.
Carter J P, Richardson D J and Spiro S 1995b Isolation and characterisation of a strain of Pseudomonas putida that can express a periplasmic nitrate reductase. Arch. Microbiol. 163, 159–166.
De Bruijn P, Van de Graaf A A, Jetten M S M, Robertson L A and Kuenen J G 1995 Growth of Nitrosomonas europaea on hydroxylamine. FEMS Microbiol Lett 125, 179–184.
Dilworth M J and Eady R R 1991 Hydrazine is a product of dinitrogen reduction by the vanadium nitrogenase from Azotobacter chroococcum. Biochem J 277, 465–468.
Gejlsbjerg B, Frette L and Westermann P 1998 Dynamics of N2O production from activated sludge. Wat. Res. 32, 2113–2121.
Gerards S, Duyts H and Laanbroek H J 1998 Ammonium-induced inhibition of ammonium-starved Nitrosomonas europaea cells in soil and sand slurries. FEMS Microbiol. Ecol. 264, 269–280.
Gupta A B 1997 Thiosphaera pantotropha – a sulphur bacterium capable of simultaneous heterotrophic nitrification and aerobic denitrification. Enzym. Microbiol. Technol. 21, 589–595.
Hellinga C, Schellen A A J C, Mulder J W, van Loosdrecht M C M and Heijnen J J 1998 The SHARON process: an innovative method for nitrogen removal from ammonium-rich waste water. Wat. Sci. Tech. 37: 135–142.
Helmer C and Kunst S 1998 Simultaneous nitrification/ denitrification in an aerobic biofilm system. Wat. Sci. Tech. 37, 83–187.
Hippen A, Rosenwinkel K H, Baumgarten G and Seyfried C F 1996 Aerobic deammonification: a new experience in the treatment of wastewaters. Wat. Sci. Tech. 35, 111–120.
Hooper A B, Vannelli T, Bergmann D J and Arciero D M 1997 Enzymology of the oxidation of ammonia to nitrite by bacteria. Antonie van Leeuwenhoek 71, 59–67.
Hovanec T A, Taylor L T, Blakis A and Delong E F 1998 Nitrospiralike bacteria associated with nitrite oxidation in freshwater aquaria. Appl. Environ. Microbiol. 64, 258–264.
Jetten M S M, Horn S J and Van Loosdrecht M C M 1997a Towards a more sustainable municipal wastewater treatment system. Wat. Sci. Tech. 35, 171–180.
Jetten M S M, Logemann S, Muyzer G M, Robertson L A, DeVries S, Van Loosdrecht M C M and Kuenen, J G 1997b Novel principles in the microbial conversion of nitrogen compounds. Antonie van Leeuwenhoek 71, 75–93.
Jetten M S M, Strous M, Van de Pas-Schoonen K T, Schalk J, Van Dongen L, Van de Graaf A A, Logemann S, Muyzer G, Van Loosdrecht M C M and Kuenen J G 1998 The anaerobic oxidation of ammonium. FEMS Microbiol. Reviews 22, 421–437
Juretschko S, Timmermann G, Schmid M, Schleifer K H, Pommerening-Roeser A, Koops H P and Wagner M 1998 Combined molecular and conventional analyses of nitrifying bacterium diversity in activated sludge – Nitrosococcus mobilis and Nitrospira-like bacteria as dominant populations. Appl. Environ. Microbiol. 64, 3042–3051.
Kester R A, Deboer W and Laanbroek H J 1997 Production of NO and N2O by pure cultures of nitrifying and denitrifying bacteria during changes in aeration. Appl. Environ. Microbiol. 63, 3872–3877.
Kuai L and Verstraete W 1998 Ammonium removal by the oxygenlimited autotrophic nitrification-denitrification system. Appl. Environ. Microbiol. 64, 4500–4506.
Laanbroek H J, Bodelier P L E and Gerards S 1994 Oxygen consumption kinetics of Nitrosomonas europaea and Nitrobacter hamburgensis grown in mixed continuous cultures at different oxygen concentrations. Arch. Microbiol. 161, 156–162.
Laanbroek, H J and Gerards, S 1993 Competition for limiting amounts of oxygen between Nitrosomonas europaea and Nitrobacter winogradskyi grown in mixed continuous cultures. Arch. Microbiol 159, 453–459.
Laanbroek H J and Woldendorp J W 1995 Activity of chemolithotrophic nitrifying bacteria under stress in natural soils. Adv. Microbial Ecol. 14, 275–304.
Logemann S, Schantl J, Bijvank S, Van Loosdrecht M C M, Kuenen J G and Jetten M S M 1998 Molecular microbial diversity in a nitrifying reactor system without sludge retention. FEMS Microbiol. Ecol. 27, 239–249.
Mulder A, Van de Graaf A A, Robertson L A and Kuenen J G 1995 Anaerobic ammonium oxidation discovered in a denitrifying fluidized bed reactor. FEMS Microbiol. Ecol. 16, 177–183.
Otte S, Grobben N G, Robertson L A, Jetten M S M and Kuenen J G 1996 Nitrous oxide production by Alcaligenes faecalis under transient and dynamic aerobic and anaerobic conditions. Appl. Environ. Microbiol. 62, 2421–2426.
Otte S, Schalk J, Kuenen J G and Jetten M S M 1999 Hydroxylamine oxidation and subsequent nitrous oxide production by the heterotrophic ammonia oxidizer Alcaligenes faecalis. Appl. Microbiol. Biotechnol. 51, 255–261.
Patureau D, Davison J, Bernet N and Moletta R 1994 Denitrification under various aeration conditions in Comamonas sp., strain SGLY2. FEMS Microbiol. Ecol. 14, 71–78.
Patureau D, Godon J J, Dabert P, Bouchez T, Bernet N, Delgenes J P and Moletta R 1998 Microvirgula aerodenitrificans gen. nov., sp. nov., A new Gram-negative bacterium exhibiting corespiration of oxygen and nitrogen oxides up to oxygen-saturated conditions. Int. J. Syst. Bacteriol. 48, 775–782.
Robertson L A, Dalsgaard T, Revsbech N P and Kuenen J G 1995 Confirmation of ‘aerobic denitrification’ in batch cultures, using gas chromatography and N-15 mass spectrometry. FEMS Microbiol. Ecol. 18, 113–119.
Schalk J, Oustad H, Kuenen J G and Jetten M S M 1998 The anaerobic oxidation of hydrazine – a novel reaction in microbial nitrogen metabolism. FEMS Microbiol. Lett. 158, 61–67.
Schmidt I and Bock E 1997 Anaerobic ammonia oxidation with nitrogen dioxide by Nitrosomonas eutropha Arch. Microbiol. 167, 106–111
Schmidt I and Bock E 1998 Anaerobic ammonia oxidation by cell free extracts of Nitrosomonas eutropha. Antonie van Leeuwenhoek 73, 271–278.
Schramm A, Debeer D, Vandenheuvel H, Ottengraf S and Amann R 1998a In situ structure/function studies in wastewater treatment systems. Wat. Sci. Tech. 37, 413–416.
Schramm A, Debeer D, Wagner M and Amann R 1998b Identification and activities in situ of Nitrosospira and Nitrospira spp. As dominant populations in a nitrifying fluidized bed reactor. Appl. Environ. Microbiol. 64, 3480–3485.
Sears H J, Ferguson S J, Richardson D J and Spiro S 1993 The identification of a periplasmic nitrate reductase in Paracoccus denitrificans. FEMS Microbiol. Lett. 113, 107–112
Siegrist H, Reithaar S and Lais P 1998 Nitrogen loss in a nitrifying rotating contractor treating ammonium rich leachate without organic carbon. Wat. Sci. Tech. 37, 589–591.
Stephen J R, Kowalchuk G A, Bruns M V, McCaig A E, Phillips C J, Embley T M and Prosser J I 1998 Analysis of beta-subgroup proteobacterial ammonia oxidizer populations in soil by denaturing gradient gel electrophoresis analysis and hierarchical phylogenetic probing. Appl. Environ. Microbiol. 64, 2958–2965.
Strous M, Van Gerven E, Ping Z, Kuenen J G and Jetten M S M 1997a Ammonium removal from concentrated waste streams with the Anaerobic Ammonium Oxidation (Anammox) process in different reactor configurations. Wat. Res. 31, 1955–1962.
Strous M, Van Gerven E, Kuenen J G and Jetten M S M 1997b Effects of aerobic and microaerobic conditions on anaerobic ammonium-oxidizing (Anammox) sludge. Appl. Environ. Microbiol. 63, 2446–2448.
Strous M, Heijnen J J, Kuenen J G and Jetten M S M 1998 The sequencing batch reactor as a powerful tool to study very slowly growing micro-organisms. Appl. Microbiol. Biotechnol. 50, 589–596.
Twachtmann U and Metzger J W 1998 A novel concept for the treatment of the effluent from anaerobic sludge digestion with trickling filters. In New advances in biological nutrient removal, Eds, D Patureau and R Moletta, pp 373–377, INRA Narbonne France.
Van Benthum W J, Garrido J M, Mathijssen J M, Sunde J, Van Loosdrecht M C M and Heijnen J J 1998 Nitrogen removal in intermittently aerated biofilm airlift reactor. J. Environ. Eng. 124, 239–248.
Van de Graaf A A, Mulder A, De Bruijn P, Jetten M S M, Robertson L A and Kuenen J G and 1995 Anaerobic oxidation of ammonium is a biologically mediated process. Appl. Environ. Microbiol. 61, 1246–1251.
Van de Graaf A A, De Bruijn P, Robertson L A, Jetten M S M and Kuenen J G 1996 Autotrophic growth of anaerobic ammonium-oxidizing micro-organisms in a fluidized bed reactor. Microbiology 142, 2187–2196.
Van de Graaf A A, De Bruijn P, Robertson L A, Jetten M S M and Kuenen J G 1997 Metabolic pathway of anaerobic ammonium oxidation on the basis of N-15 studies in a fluidized bed reactor. Microbiology 143, 2415–2421.
Van Loosdrecht M C M and Jetten M S M 1998 Microbiological conversions in nitrogen removal. Wat. Sci. Tech. 38, 1–7.
Van Luijn F, Boers P C M and Lijklema L 1998 Anoxic N2 fluxes from freshwater sediments in the absence of oxidized nitrogen compounds. Wat. Res 32, 407–409.
Zart D and Bock, E 1998 High rate aerobic nitrification and denitrification by Nitrosomonas eutropha grown in a fermentor with complete biomass retention in the presence of NO2 and NO. Arch. Micrbiol. 169, 282–286.
Zumft WG 1997 Cell biology and molecular basis of denitrification. Microbiol. Mol. Biol. Rev. 61, 533–568 Section editor: H Lambers
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Jetten, M.S. New pathways for ammonia conversion in soil and aquatic systems. Plant and Soil 230, 9–19 (2001). https://doi.org/10.1023/A:1004683807250
Issue Date:
DOI: https://doi.org/10.1023/A:1004683807250