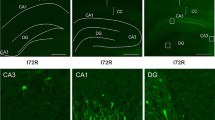
Abstract
We investigated early alterations in rat neurons after experimental ischemic stress. Transient ischemia was generated by bilateral occlusion of the carotids after hypoxia. Data show a relevant increase of the nuclear level of ubiquitin 2 h post-stress as evaluated by immuno-cytolocalization. Ubiquitin returns to normal levels after 6 h. The increase in ischemic/hypoxic rats was localized preferentially in nuclei of hippocampal neurons, although some augmentation was also shown essentially in dendrites. The activation of ubiquitin system is related to a defective homeostasis and might trigger different degenerative processes. With respect to this, we observed chromatin alterations by densitometric analysis. The shown extensive DNA degeneration is consistent with the occurrence of necrotic phenomena at an early stage. However the parallel internucleosomal specific DNA fragmentation, strongly suggests that apoptotic events also occur. In any case both necrosis and apoptosis are likely to occur at same time, although apoptosis is less extensive, and two phenomena take place in different neural cells.
Similar content being viewed by others
References
Hershko A, Ciechanower A: The ubiquitin system for protein degradation. Ann Rev Biochem 61: 761-807, 1992
Ciechanower A, Schwartz AL: The ubiquitin-mediated proteolytic pathway: Mechanism of recognition of the proteolytic substrate and involvement in the degradation of native cellular proteins. Faseb J 8: 182-191, 1994
Kirino T: Delayed neuronal death in the gerbil hippocampus following ischemia. Brain Res 239: 57-69, 1982
Pulsinelli WA, Brierley JB, Plum F: Temporal profile of neuronal damage in a model of transient forebrain ischemia. Ann Neurol 11: 491-498, 1982
Smith ML, Auer RN, Siesjo BK: The density and distribution of ischemic brain injury in the rat following 2–10 min of forebrain ischemia. Acta Neuropathol 64: 319-332, 1984
Wieloch T: Neurochemical correlates to selective neuronal vulnerability. Prog Brain Res 63: 69-85, 1985
Petito CK, Feldmann E, Pulsinelli WA, Plum F: Delayed hippocampal damage in humans following cardiorespiratory arrest. Neurology 37: 1281-1286, 1987
Leoni S, Brambilla D, Risuleo G, de Feo G, Scarsella G: Effect of different whole body hypertermic sessions on the heat shock response in mice liver and brain. Mol Cell Biochem 204: 41-47, 2000
Liu Y, Kato H, Nakata N, Kogure K: Temporal profile of heat shock protein 70 synthesis in ischemic tolerance induced by preconditioning ischemia in rat hippocampus. Neuroscience 56: 921-927, 1993
Kato H, Liu Y, Kogure K, Kato K: Induction of 27-kDa heat shock protein cerebral ischemia in a rat model of ischemic tolerance. Brain Res 63: 69-85, 1994
Torregrosa G, Barbera MD, Orti M, Centeno JM, Salom JB, Justicia C, Planas AM, Alborch E: Temporospatial expression of HSP72 and c-JUN, and DNA fragmentation in goat hippocampus after global cerebral ischemia. Hippocampus 11: 146-56, 2001
Hayashi T, Takada K, Matsuda M: Post transient ischemia increase in ubiquitin conjugates in the early reperfusion. Neuroreport 3: 519-520, 1992
Hayashi T, Takada K, Matsuda M: Subcellular distribution of ubiquitin-protein conjugates in the hippocampus following transient ischemia. J Neurosci Res 31: 561-564, 1992
Hayashi T, Tanaka J, Kamikubo T, Takada K, Matsuda M: Increase in ubiquitin conjugates dependent on ischemic damage. Brain Res 620: 171-173, 1993
Kamikubo T, Hayashi T, Ohkawa K: Lack of effect of transient ischemia on ubiquitin conjugation. Neurochem Res 20: 391-394, 1995
Gubellini P, Bisso GM, Ciofi-Luzzato A, Fortuna S, Lorenzini P, Michalek H, Scarsella G: Ubiquitin-mediated stress response in a rat model of brain transient ischemia/hypoxia. Neurochem Res 22: 93-100, 1997
Mengual E, Aritzi P, Rodrigo J, Gimenezamaja JM, Castano JG: Immunohistochemical distribution and electron microscopic subcellular localization of the proteasome in the rat CNS. J Neurosci 16: 6331-6341, 1996
Davies N, Lindsey GG: Histone H2B and H2A ubiquitination allow normal octamer and core particle reconstitution. Biochem Biophys Acta 1218: 187-193, 1993
Finley D, Chau V: Ubiquination. Annu Rev Cell Biol 7: 66-69, 1991
Hirt B: Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol 26: 708-714, 1967
Cristofanilli M, Iacoangeli A, Risuleo G, Scarsella G: A simple and rapid method allowing partial purification of low molecular weight DNA. Recent Res Dev Biotech Bioeng 4: 1-5, 2001
Bresin A, Iacoangeli A, Risuleo G, Scarsella G: Ubiquitin dependent proteolysis is activated in apoptotic fibroblasts in culture. Mol Cel Biochem 220: 57-60, 2001
Graham SH, Chen J: Programmed cell death in cerebral ischemia. J Cereb Blood Flow Metab 21: 99-109, 2001
Caday CG, Sklar RM, Berlowe D Jr, Kemmou A, Brown RH Jr, Finklestein SP: Poliubiquitin gene expression following cerebral ischemia. Ann NY Acad Sci 679: 188-194, 1993
Cao G, Pei W, Lan J, Stetler RA, Luo Y, Nagayama T, Graham SH, Yin XM, Simon RP, Chen J: Caspase-activated DNase/DNA fragmentation factor 40 mediates apoptotic DNA fragmentation in transient cerebral ischemia and in neuronal cultures. J Neurosci 21: 4678-90, 2001
Zhan RZ, Wu C, Fujihara H, Taga K, Qi S, Naito M, Shimoji K: Both caspase-dependent and caspase-independent pathways may be involved in hippocampal CA1 neuronal death because of loss of cytochrome c from mitochondria in a rat forebrain ischemia model. J Cereb Blood Flow Metab 21: 529-540, 2001
Canese R, Fortuna S, Lorenzini P, Podo F, Michalek H: Transient global ischemia in the rat: Spatial distribution, extension and evolution of lesion evaluated by magnetic resonance imaging. MAGMA 7: 28-34, 1998
Picologlou S, Brown N, Liebman SW: Mutations in RAD6, a yeast gene encoding a ubiquitin-conjugating enzyme, stimulate retrotransposition. Mol Cell Biol 10: 1017-1022, 1990
Tsukada T, Watanabe M, Yamashima T: Implications of CAD and DNase II in ischemic neuronal necrosis specific for the primate hippocampus. J Neurochem 79: 1196-1206, 2001
Yao H, Takasawa R, Fukuda K, Shiokawa D, Sadanaga-Akiyoshi F, Ibayashi S, Tanuma S, Uchimura H: DNA fragmentation in ischemic core and penumbra in focal cerebral ischemia in rats. Brain Res Mol Brain Res 91: 112-118, 2001
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Risuleo, G., Cristofanilli, M. & Scarsella, G. Acute ischemia/hypoxia in rat hippocampal neurons activates nuclear ubiquitin and alters both chromatin and DNA. Mol Cell Biochem 250, 73–80 (2003). https://doi.org/10.1023/A:1024950317684
Issue Date:
DOI: https://doi.org/10.1023/A:1024950317684