Abstract
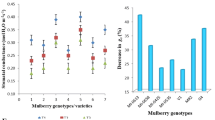
The effect of water stress on photosynthesis was determined in five mulberry cultivars (Morus alba L. cv. K-2, MR-2, BC2-59, S-13 and TR-10). Drought was imposed by withholding water and the plants were maintained at different water potentials ranging from 0.5 -MPa to 2.0 -MPa. Photosynthetic rates, activities of ribulose-1,5-bisphosphate carboxylase and sucrose phosphate synthase, photosystem II activity and chlorophyll content were used as key parameters to assess photosynthetic performance. There was a marked variation in the photosynthetic rates and ribulose-1,5-bisphosphate carboxylase activity among the five mulberry cultivars subjected to water stress. Photosystem II (PSII) and sucrose phosphate synthase activities were also severely reduced as measured by drought conditions. Of the five mulberry cultivars, S-13 and BC2-59 showed higher photosynthetic rates, ribulose-1,5-bisphosphate carboxylase activity, high sucrose phosphate synthase activity and photochemical efficiency of PSII compared to the other varieties.
Similar content being viewed by others
References
Arnon D.I. 1949. Copper enzymes in isolated chloroplasts. Polyphenol oxidases in Beta vulgaris. Plant Physiol. 24: 1–15.
Asada K. 1996. Radical production and scavenging in the chloroplasts. In: Baker N.R. (ed.), Photosynthesis and the Environment. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp. 123–150.
Bjorkman O. and Powles S.B. 1984. Inhibition of photosynthetic reactions under water stress: Interaction with the light level. Planta 161: 490–504.
Boyer T.S., Wong S.C. and Farquhar G.D. 1997. CO2 and water vapour exchange across the leaf cuticle (epidermis) at various water potentials. Plant Physiol. 114: 185–189.
Chaitanya K.V., Sundar D., Masilamani S. and Ramachandra Reddy A. 2002. Variation in heat stress-induced antioxidant enzyme activities among three mulberry cultivars. Plant Growth Regul. 36: 175–180.
Dubois M., Gilles K.A., Hamilton J.K., Rebers P.A. and Smith F. 1956. Colorimetric method for the determination of sugars and related substances. Anal. Biochem. 28: 350–356.
Foyer C.H., Descourvieres P. and Kunert K.J. 1994. Protection against oxygen radicals: an important defense mechanism studied in transgenic plants. Plant Cell Environ. 17: 507–523.
Fryer M.J., Andrews J.R., Oxborough K., Blowers D.A. and Baker N.R. 1998. Relationship between CO2 assimilation, photosynthetic electron transport and active O2 metabolism in the leaves of maize in the field during the periods of low temperature. Plant Physiol. 116: 571–580.
Griffiths H. and Parry M.A.J. 2002. Plant responses to water stress. Ann. Bot. 89: 801–802.
Huber S.C. 1981. Interspecific variation in the activity and regulation of leaf sucrose phosphate synthase. Z. Pflanzenphysiol. 102: 443–450.
Iturbe-Ormaetxe I.I., Escuredo P.R., Arrese-Igor C. and Becana M. 1998. Oxidative damage in pea plants exposed to water deficit or paraquat. Plant Physiol. 116: 173–181.
Kaiser W.M. 1987. Effects of water deficit on photosynthetic capacity. Physiol. Plant. 71: 142–149.
Leegood R.C. and Walker D.A. 1993. Chloroplasts and protoplasts. In: Hall D.O., Scurlock J.M.O., Bolharnordenkampf H.R., Leegood R.C. and Long S.P. (eds), Photosynthesis and Production in Changing Environment-a Field and Laboratory Manual. Chapman and Hall, London, pp. 268–282.
Liley R., Mc C., Fitzergald M.P., Rienitis K.G. and Walker D.A. 1975. Criteria of intactness and photosynthetic activity of spinach chloroplast preparations. New Phytol. 75: 1–10.
Lawlor D.W. 2002. Limitation to photosynthesis in water-stressed leaves: stomata vs metabolism and the role of ATP. Ann. Bot. 89: 871–885.
Lawlor D.W. and Cornic G. 2002. Photosynthetic carbon assimilation and associated metabolism in relation to water deficits in higher plants. Plant Cell Environ. 25: 275–294.
Lorimer G.H., Badger M.R. and Andrews T.J. 1977. D-ribulose-1,5-bisphosphate carboxylase-oxygenase. Improved methods for activation and assay of catalytic activities. Anal. Biochem. 78: 66–75.
Maroco J.P., Edwards G.E. and Ku M.S.B. 1999. Photosynthetic acclimation of maize to growth under elevated levels of carbon dioxide. Planta 1210: 115–125.
Noctor G., Veljovic-Jovanovic S. and Foyer C.H. 2000. Peroxide processing in photosynthesis: antioxidant coupling and redox signalling. Proc. Royal Soc. London B355: 1465–1475.
Noctor G., Veljovic-Jovanovic S., Driscoll S., Novitskaya L. and Foyer C.H. 2002. Drought and oxidative load in the leaves of C3 plants: a predominant role for photorespiration. Ann. Bot. 89: 841–850.
Osmond B., Badger M., Maxwell K., Bjorkman O. and Leegood R. 1997. Too many photons: photorespiration, photoinhibition and photooxidation. Trends Plant Sci. 2: 119–120.
Raghavendra A.S. and Das V.S.R. 1976. Distribution of C4 dicarboxylic acid pathway of photosynthesis in local monocotyledonous plants and its taxonomic significance. New Phytol. 76: 301–305.
Ramachandra Reddy A., Reddy K.R. and Hodges H.F. 1996. Mepiquat chloride (PIX)-induced changes in photosynthesis and growth of cotton. Plant Growth Regul. 20: 179–183.
Ramachandra Reddy A., Chaitanya K.V. and Sundar D. 2000. Water stress mediated changes in antioxidant enzyme activities of mulberry (Morus alba L). J. Seric. Sci. Japan 69: 169–175.
Sundar D. and Ramachandra Reddy A. 2000. Low night temperature-induced changes in photosynthesis and rubber accumulation in Guayule (Parthenium argentatum Gray). Photosynthetica 38: 421–427.
Tezara W., Mitchell V.J., Driscoll S.D. and Lawlor D.W. 1999. Water stress inhibits plant photosynthesis by decreasing coupling factor and ATP. Nature 401: 914–917.
Vassey T.L. and Sharkey T.D. 1989. Mild water stress of Phaseolus vulagris leads to reduced starch synthesis and extractable sucrose phosphate synthase activity. Plant. Physiol. 189: 1066–1070.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Chaitanya, K., Jutur, P., Sundar, D. et al. Water stress effects on photosynthesis in different mulberry cultivars. Plant Growth Regulation 40, 75–80 (2003). https://doi.org/10.1023/A:1023064328384
Issue Date:
DOI: https://doi.org/10.1023/A:1023064328384