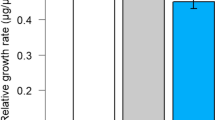
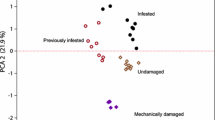
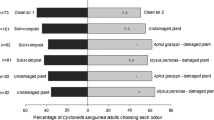
Abstract
The response of the bird cherry-oat aphid, Rhopalosiphum padi, to barley plants was investigated following exposure of the plants to root allelochemicals from the aggressive weed couch-grass, Elytrigia (Agropyron) repens. Plants were treated either with root exudates from living couch-grass plants or with previously identified couch-grass root compounds [5-hydroxyindole-3-acetic acid, DL-5-hydroxytryptophan, L-5-hydroxytryptophan hydrate, and 6-hydroxy-1,2,3,4-tetrahydro-β-carboline-3-carboxylic acid (carboline)] either separately or in mixtures. In choice and no-choice settling tests, aphid acceptance of barley plants was significantly reduced following treatment with root exudates, and the carboline when tested alone or in combination with the other compounds. In contrast, the other compounds without the carboline were less active in reducing aphid acceptance. In a probing bioassay, individual substances were either neutral or stimulatory to aphids, indicating that the reduced settling was probably not due to direct effects on aphids, but rather due to effects on the plant. This was confirmed in olfactometer assays, in which aphids were repelled by odors from barley plants following treatment with a mixture containing all four chemicals.
Similar content being viewed by others
References
åhman, I., Tuvesson, S., and Johansson, M. 2000. Does indole alkaloid gramine confer resistance in barley to aphid Rhopalosiphum padi? J. Chem. Ecol. 26:233–255.
Bobnick, S. J. and Hagin, R. D. 1985. Allelopathic effects of vanillin and its glycoside on germinating legume seeds. Proceedings, 39th annual meeting Northeastern Weed Science Society, p. 76.
Brossi, A., Focella, A., and Teitel, S. 1973. Alkaloids in mammalian tissues. 3. Condensation of l-tryptophan and l-5–hydroxytryptophan with formaldehyde and acetaldehyde. J. Med. Chem. 16:418–420.
Forslund, K., Pettersson, J., Ahmed, E., and Jonsson, L. 1998. Settling behaviour of Rhopalosiphum padi (L.) in relation to cyanogenic glycosides and gramine contents in barley. Acta Agric. Scand. B, Soil Plant Sci. 48:107–112.
Friebe, A., Schulz, M., Kueck, P., and Schnabl, H. 1995. Phytotoxins from shoot extracts and root exudates of Agropyron repens seedlings. Phytochemistry 38:1157–1159.
Hagin, R. D. 1989. Isolation and identification of 5–hydroxyindoleacetic acid and 5–hydroxytryptophan, major allelopathic aglycons in quackgrass (Agropyron repens, L. Beauv.). J. Agric. Food Chem. 37:1143–1149.
Hagin, R. D. and Bobnick, S. J. 1991. Isolation and indentification of a slug-specific molluscicide from quackgrass (Agropyron repens, L. Beauv.). J. Agric. Food. Chem. 39:192–196.
Karban, R., Baldwin, I. T., Baxter, K. J., Laue, G., and Felton, G. W. 2000. Communication between plants: induced resistance in wild tobacco plants following clipping of neighboring sagebrush. Oecologia 125:66–71.
Liu, D. L. and Lovett, J. V. 1993. Biologically active secondary metabolites of barley II. Phytotoxicity of barley allelochemicals. J. Chem. Ecol. 19:2231–2244.
Lovett, J. V., Hoult, A. H. C., Inderjit, Dakshini, K. M. M., and Einhellig, F. A. 1995. Allelopathy and self-defense in barley, pp. 170–183, in Inderjit and K. M. M. Dakshini (Eds.). Allelopathy: Organisms, Processes and Applications. American Chemical Society Symposium Series No. 582, Washington, D.C.
Muller, C. H. 1969. Allelopathy as a factor in ecological process. Vegetatio 18:348–357.
Ninkovic, V., Olsson, U., and Pettersson, J. 2002. Mixing barley cultivars affects aphid host plant acceptance in field experiments. Entomol. Exp. Appl. 102:177–182.
Oswald, H. 1948. Toxic exudates from the roots of Agropyron repens. J. Ecol. 38:192–193
Pettersson, J. 1970. Studies on Rhopalosiphum padi (L.). Laboratory studies on olfactometric responses to winter host Prunus padus L. Lantbrukshoegsk. Ann. 36:381–399.
Pettersson, J. 1994. The bird cherry-oat aphid, Rhopalosiphum padi (HOM.: APH.) and odours, pp. 3–12, in S. R. Leather, A. Wyatt, N. A. C. Kidd, and K. F. A. Walters (Eds.). Individuals, Populations and Patterns in Ecology. Intercept Ltd., Andover, United Kingdom.
Pettersson, J., Quiroz, A., and Ahmed, F. E. 1996. Aphid antixenosis mediated by volatiles in cereals. Acta Agric. Scand. B, Soil Plant Sci. 46:135–140.
Pettersson, J., Ninkovic, V., and Ahmed, A. 1999. Volatiles from different barley cultivars affect aphid acceptance of neighbouring plants. Acta. Agric. Scand. B. Soil Plant Sci. 49:152–157.
Pickett, J. A., Wadhams, L. J., Woodcock, C. M., and Hardie, J. 1992. The chemical ecology of aphids. Annu. Rev. Entomol. 37:67–90.
Phlak, F. 1967. The effect of quackgrass on succeeding plants. Plant Soil 27:272–384.
Prado, E. and Tjallingii, W. F. 1997. Effects of previous plant infestation on sieve element acceptance by two aphids. Entomol. Exp. Appl. 82:189–200.
Reigosa, M. J., Sánchez-Moreiras, A., and González, L. 1999. Ecophysiological approach to allelopathy. Crit. Rev. Plant. Sci. 18:577–608.
Rice, E. L. 1984. Allelopathy, 2nd ed. Academic Press, New York.
Sandström, J., Nieto-Nafria, J. M., and Dixon, A. F. G. 1998. Nutritional quality of phloem sap in relation to aphid host plant use. Proceedings, 5th International Sympsiumon Aphids, Leon, Spain, September, 1997. pp. 265–269.
SAS. 1997. SAS/STAT Software: Changes and Enhancements Through Release 6 12. SAS Institute, Cary, North Carolina.
Schulz, M., Friebe, A., Kueck, P., Seipel, M., and Schnabl, H. 1994. Allelopathic effects of living quackgrass (Agropyron repens L.): identification of inhibitory allelochemicals exuded from rhizome borne roots. Angew. Bot. 68:195–200.
Slesak, E., Slesak, M., and Gabrys, B. (2001) Effect of methyl jasmonate on hydroxamic acid content, protease activity, and bird cherry-oat aphid Rhopalosiphum padi (L.) probing behavior. J. Chem. Ecol. 27:2529–2543.
Touchette, R., Leroux, G. D., and Deschenes, J. M. 1988. Allelopathic activity of quackgras (Agropyron repens) extracts and residues on alfalfa (Medicago sativa). Can. J. Plant Sci. 68:785–792.
Velozo, J. A., Alvarez, R. I., Wachter, G. A., Timmermann, B. N., and Corcuera, L. J. 1999. Increase in gramine content in barley infested by the aphid Schizaphis graminum R. Phytochemistry 52:1059–1061.
Welbank, P. J. 1960. Toxin production from Agropyron repens, pp. 158–164, in J. L. Harper (ed.). The Biology of Weeds. Blackwell Scientific Publications, Oxford, United Kingdom.
Weston, L. A. and Putnam, A. R. 1985. Inhibition of growth, nodulation and nitrogen fixation of legumes by quackgrass. Crop Sci. 25:561–565.
Weston, L. A. and Putnam, A. R. 1986. Inhibition of legume seedling growth by residues and extracts of quackgrass (Agropyron repens). Weed Sci. 34:366–372.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Glinwood, R., Pettersson, J., Ahmed, E. et al. Change in Acceptability of Barley Plants to Aphids After Exposure to Allelochemicals from Couch-Grass (Elytrigia repens). J Chem Ecol 29, 261–274 (2003). https://doi.org/10.1023/A:1022687025416
Issue Date:
DOI: https://doi.org/10.1023/A:1022687025416