Abstract
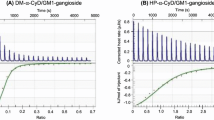
The interaction between glycosphingolipids and recombinant human GM2-activator was studied in a microwell binding assay. A-series gangliosides like GM3, GM2 and GM1 were strongly bound by the recombinant human GM2 activator. A weak binding was observed to GD1b and sulfatide, while neutral glycolipids were not bound. Optimal binding occurred at pH 4.2 and was inhibited by increasing concentrations of citrate buffer and NaCl. In contrast with these in vitro results the recombinant human GM2-activator is able to restore the degradation of GA2 in fibroblasts from patients with the AB variant of GM2 gangliosidosis in vivo.
Similar content being viewed by others
REFERENCES
Leeden, R. W., and Yu, R. K. 1982. Gangliosides: Structure, Isolation, and Analysis. Pages 139–189, in Ginsburg (ed.), Methods in Enzymology Vol. 83, Academic Press, New York.
Sandhoff, K., and van Echten, G. 1993. Ganglioside Metabolism-Topology and Regulation. Pages 119–142 in Bell, R. M., Merrill, A. H., and Hannun, Y. A. (eds.), Advances in Lipid Research Vol. 26, Academic Press, New York.
Sandhoff, K., and Kolter, T. 1996. Topology of glycosphingolipid degradation. Trends in Cell Biology 6:98–103.
Fürst, W. and, Sandhoff, K. 1992. Activator proteins and topology of lysosomal sphingolipid catabolism. Biochim. Biophys. Acta 1126:1–16.
Sandhoff, K., Harzer, K., and Fürst, W. 1995. Sphingolipid activator proteins. Pages 2427–2441, in Scriver, C. R., Beaudet, A. L., Sly, W. S., and Valle, D. (eds), The Metabolic and Molecular Bases of Inherited Disease, 7th edn., Vol. 2, McGraw Hill, New York.
Kytzia, H.-J., and Sandhoff, K. 1985. Evidence for two different active sites on human hexosaminidase A. J. Biol. Chem. 260:7568–7572.
Conzelmann, E., and Sandhoff, K. 1979. Purification and characterization of an activator protein for the degradation of glycolipids GM2 and GA2 by hexosaminidase A. Hoppe-Seyler's Z. Physiol. Chem. 360:1837–1849.
Li, S.-C., Hirabayashi, Y., and Li, Y.-T. 1981. A protein activator for the enzymic hydrolysis of GM2 ganglioside. J. Biol. Chem. 256:6234–6240.
Sandhoff, K., Conzelmann, E., Neufeld, E. F., Kaback, M. M., and Suzuki, K. 1989. The GM2-Gangliosidosis. Pages 1807–1839, in Scriver, C: R., Beaudet, A. L., Sly, W. S. and Valle, D. (eds.), The Metabolic Basis of Inherited Diseases, 6th edn., Vol. 2, McGraw-Hill, New York.
Sonderfeld, S., Conzelmann, E., Schwarzmann, G., Burg, J., Hinrichs, U., and Sandhoff, K. 1985. Incorporation and metabolism of ganglioside GM2 in skin fibroblasts from normal and GM2 gangliosidosis subjects. Eur. J. Biochem. 149:247–255.
Conzelmann, E., Burg, J., Stephan, G., and Sandhoff, K. 1982. Complexing of glycolipids and their transfer between membranes by the activator protein for degradation of lysosomal ganglioside GM2. Eur. J. Biochem. 123:455–464.
Fürst, W., Schubert, J., Machleidt, W., Meyer, H. E., and Sandhoff, K. 1990. The complete amino-acid sequence of human ganglioside GM2-activator protein and cerebroside sulfate activator. Eur. J. Biochem. 192:709–714.
Klima, H., Klein, A., van Echten, G., Schwarzmann, G., Suzuki, K., and Sandhoff, K. 1993. Over-expression of a functionally active human GM2-activator protein in Escherichia coli. Biochem. J. 292:571–576.
Kasai, N., Sillerud, L. O., and Yu, R. K. 1982. A convenient method for the preparation of asialo-GM1. Lipids 17:107–110.
Klein, A., Henseler, M., Klein, C., Suzuki, K., Harzer, K., and Sandhoff, K. 1994. Sphingolipid activator protein D (sap-D) stimulates the lysosomal degradation of ceramide in vivo. Biochem. Biophys. Res. Commun. 200:1440–1448.
Childs, R. A., Wright, J. R., Ross, G. F., Yuen C-T., Lawson, A. M., Chai, W., Drickamer, K., and Feizi, T. 1992. Specificity of lung surfactant protein SP-A for both the carbohydrate and the lipid moieties of certain neutral glycolipids. J. Biol. Chem. 267:9972–9979.
Schepers, U., Glombitza, G., Lemm, T., Hoffmann, A., Chabas, A., Ozand, P., and Sandhoff, K. 1996. Molecular analysis of a GM2-activator deficiency in two patients with GM2-gangliosidosis AB variant. Am. J. Hum. Genet. 59:1048–1056.
Kyrklund, T. 1987. Two procedures to remove polar contaminants from a crude brain lipid extract by using prepacked reversed-phase columns. Lipids 22:274–277.
Hama, Y., Li, Y.-T., and Li S.-C. 1997. Interaction of GM2 activator protein with glycosphingolipids. J. Biol. Chem. 272:2828–2833.
Sandhoff, K., Harzer, K., Wässle, W., and Jatzkewitz, H. 1971. Enzyme alterations and lipid storage in three variants of Tay-Sachs disease. J. Neurochem. 18:2469–2489.
Liu, Y., Hoffmann, A., Grinberg, A., Westphal, H., McDonald, M. P., Miller, K. M., Crawley, J. N., Sandhoff, K., Suzuki, K., and Proia, R. L. 1997. Mouse model of GM2 activator deficiency manifests cerebellar pathology and motor impairment. Proc. Natl. Acad. Sci. USA 94:8138–8143.
Meier, E., Schwarzmann, G., Fürst, W., and Sandhoff, K. 1991. The human GM2-activator protein: a substrate specific cofactor of hexosaminidase A. J. Biol. Chem. 266:1879–1887.
Li, S.-C., Serizawa, S., Li, Y.-T., Nakamura, K., and Handa, S. 1984. Effect of modification of sialic acid on enzymic hydrolysis of gangliosides GM1 and GM2. J. Biol. Chem. 259:5409–5410.
Wu, Y. W., Lockyer, J. M., Sugiyama, E., Pavlova, N. V., Li, Y.-T., and Li, S.-C. 1994. Expression and specificity of human GM2 activator protein. J. Biol. Chem. 269:16276–16283.
Paton, B. C., Schmid, B., Kustermann-Kuhn, B., Poulos, A., and Harzer, K. 1992. Additional biochemical findings in a patient and fetal sibling with a genetic defect in the sphingolipid activator protein (SAP) precusor, prosaposin. Evidence for a deficiency in SAP-1 and for a normal lysosomal neuraminidase. Biochem. J. 285:481–488.
Bradova, V., Smid, F., Ulrich-Bott, B., Roggendorf, W., Paton, B. C., and Harzer, K. 1993. Prosaposin deficiency: further characterization of the sphingolipid activator protein-deficient sibs. Multiple glycolipid elevations (including lactosylceramidosis), partial enzyme deficiencies and ultrastructure of the skin in this generalized sphingolipid storage disease. Hum. Genet. 92:143–152.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bierfreund, U., Lemm, T., Hoffmann, A. et al. Recombinant GM2-Activator Protein Stimulates In Vivo Degradation of GA2 in GM2 Gangliosidosis AB Variant Fibroblasts But Exhibits No Detectable Binding of GA2 in an In Vitro Assay. Neurochem Res 24, 295–300 (1999). https://doi.org/10.1023/A:1022526407855
Issue Date:
DOI: https://doi.org/10.1023/A:1022526407855