Abstract
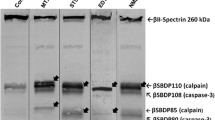
Analyses using either one or two-dimensional gel electrophoresis were performed to identify the contribution of several proteases to lower molecular weight (MW) neurofilament 68 (NF68) break down products (BDPs) detected in cortical homogenates following unilateral cortical impact injury in rats. One dimensional immunoblot of BDPs obtained from in vitro cleavage of enriched neurofilaments (NF) by purified μ-calpain, m-calpain, cathepsin, B, cathepsin D, and CPP32 (caspase-3) were compared to in vivo samples from rats following traumatic brain injury (TBI). Comparison of these blots provided information on the relative contribution of different cysteine or aspartic proteases to NF loss following brain injury. As early as 3 hrs post-injury, cortical impact resulted in the presence of several lower MW NF68 immunopositive bands having patterns similar to those previously reported to be produced by calpain mediated proteolysis of neurofilaments. Only μ-calpain and m-calpain in vitro digestion of enriched neurofilaments contributed to the presence of the low MW 57 kD NF68 break down product (BDP) detected in post-TBI samples. Cathepsin B, cathepsin D, and caspase-3 failed to produce either the 53 kD or 57 kD NF BDPs. Further, 1 and 2 dimensional peptide maps containing a 1:1 ratio of in vivo and in vitro tissue samples showed complete comigration of lower MW immunopositive spots produced by TBI or in vitro incubation with m-calpain, thus providing additional evidence for the potential role of calpain activation to the production of NF68 BDPs following TBI. More importantly, 2-dimensional gel electrophoresis detected that immunopositive NF68 spots shifted to the basic pole (+) suggesting that dephosphorylation of the NF68 subunit pool may be associated with NF protein loss following TBI, an observation not previously noted in any model of experimental brain injury.
Similar content being viewed by others
REFERENCES
Schlaepfer, W., Lee, C., Trojanowski, J., and Lee, V. M. 1984. Persistence of immunoreactive neurofilament protein breakdown products in transfected rat sciatic nerve. J. Neurochem 43:857-864.
Schlaepfer, W., Lee, W., Lee, C., and Zimmerman, U. P. 1985. An immunoblot study of neurofilament degradation in situ and during calcium activated proteolysis. J Neurochem 44:502-509.
Schlaepfer, W. and Zimmerman, U. P. 1990. Degradation of neurofilaments by neutral calcium activated proteases. In: CRC's Intracellular calcium dependent proteolysis (Mellegren RL, Murachi, ed), pp. 241-248.
Hong, S. C., Lazino, G., Goto, S., Kang, S. K., Schottler, F., Kassell, N. F., and Lee, K. S. 1994. Calcium activated proteolysis in rat neocortex induced by transient focal ischemia. Brain. Res. 661:43-50.
Hong, S. C., Goto, Y., Lanzino, G., Soleau, S., Kassell, N. F., and Lee, K. S. 1995. Neuroprotection with a calpain inhibitor in a model of focal cerebral ischemia. Stroke, 25:663-669.
Banik, N. L., Hogan E. L., Powers, J. M., and Whetstine, L. 1982. Degradation of cytoskeletal proteins in experimental spinal cord injury. Neurochem. Res. 7:1465-1475.
Hall, E. D. 1985. Beneficial effects of acute intravenous ibuprofen on neurological recovery of head-injured mice: Comparison of cyclooxygenase inhibitors with inhibition of thromboxane A2 synthetase. Central Nervous System Trauma. 2:75-83.
Posmantur, R., Hayes, R. L., Dixon, C. E., and Taft, W. C. 1994. Neurofilament 68 and neurofilament 200 protein levels decrease after traumatic brain injury. J. Neurotrauma, 11:533-545.
Posmantur, R. M., Kampfl, A., Liu, S. J., Heck, K., Taft, W. C. Clifton, G. L., and Hayes, R. L. 1996. Cytoskeletal derangements of cortical neuronal processes three hours after traumatic brain injury in rats: an immunofluorescence study. J. Neuropath. Exp. Neurol. 55:68-90.
Posmantur, R., Kampfl, A., Siman, R., Liu, S. J., Zhao, X., Clifton, G. L., and Hayes, R. L. 1997. A calpain inhibitor attenuates cortical cytoskeletal protein loss after experimental traumatic brain injury in the rat. Neuroscience 77:875-888.
Posmantur, R., Kampfl, A., Taft, W. C., Bhattacharjee, M., Dixon, C. E., Bao, J., and Hayes, R. L. 1996b. Diminished microtubule associated protein 2 immunoreactivity following cortical impact injury. J. Neurotrauma 13:125-137.
Hicks, R., Douglas, S. H., and McIntosh, T. K. 1995. Temporal response and effects of excitatory amino acid antagonism on microtubule associated protein 2 immunoreactivity following experimental brain injury in rats. Brain Res., 678:151-160.
Taft, W. C., Yang, K., Dixon, C. E., Clifton, G. L., and Hayes, R. L. 1993. Hypothermia attenuates the loss of hippocampal microtubule-associated protein 2 (MAP2) following traumatic brain injury. J Cereb. Blood. Flow. Metab. 13:796-802.
Saatman, K. E., Bozyczko-Coyne, D., Siman, R., Marcy, V., Generalli, A., and McIntosh, T. K. 1995. Calpain I activation following experimental brain injury. J. Neuropath. Exp. Neurol. 55:852-862.
Newcomb, J. K., Liu, S. J., Kampfl, A., Zhao, X., Posmantur, R., Clifton, G. L., and Hayes, R. L. 1997. Examination of of calpain 1 specific break-down products to α-spectrin in a controlled cortical impact model at both early and late timepoints. J. Neurotrauma 6:369-383.
Zhao, X., Posmantur, R., Liu, J. S., Kampfl, A., Clifton, G. L., and Hayes, R. L. 1997. A quantitative measure of calpain activity following traumatic brain injury using casein zymography. Journal of Cerebral Blood Flow Metab. In Press.
Hill, I. E., Preston, E., Monette, R., and MacManus, J. P. 1997. A comparison of cathepsin B processing and distribution during neuronal death in rats following gloal ischemia or decapitation necrosis. Brain Res.; 751:206-216.
Kohda, Y., Yamashima, T. R., Sakuda, K., Yamashita, J., Ueno, T., Kominami, E., and Yoshiokoka, T. 1996. Dynamic changes of cathepsin B and L expression in the monkey hippocampus after transient ischemia. Biochem. Biophys. Acta. 228:616-622.
Nitatori, T., Sato, N., Kominami, E., Uchiyama, Y. 1996. Participation of cathepsins B, H, and L in perikaryal condensation of CA1 pyramidal neurons undergoing apoptosis after brief ischemia. J. Neurotrauma 6:369-383.
Alnemri, E. S., Livingston, D. J., Nicholson, D. W., Salvesen, G., Thornberry, N. A., Wong, W. W., and Yuan, J. 1996. Human ICE/CED-3 protease nomenclature. Cell. 87:171.
Fraser, A. and Evan, G. 1996. A licence to kill. Cell. 85:781-784.
Nath, R., Raser, K. J., Stafford, D., Hajimohammadreza, I., Posner, A., Allen, H., Talanian, R., Yuen, P. Y., Gilbertsen, R. B., and Wang, K. W. 1996. Non-erythoid α-spectrin BDP by calpain and interleukin 1B-converting enzyme like proteases in apoptotic cells: contributory roles of both protease families in neuronal apoptosis. Biochem J. 318:683-690.
Posmantur, R., McGinnis, K., Nadimpalli, R., Gilbertsen, R. B., and Wang, K. K. W. 1997b. Characterization of CPP32-like protease activity following apoptotic challenge in SH-SY5Y neuroblastoma cells. J. Neurochemistry 68:2328-2337.
Taft, W. C., Yang, K., Dixon, C. E., and Hayes, R. L. 1992. Microtubule associated protein 2 level in hippocampus following traumatic brain injury. J. Neurotrauma 9:281-290.
Nilsson, P., Hillered, L., Ponten, U., and Ungerstedt, Y. 1990. Changes in cortical extracellular levels of energy related metabolites and amino acids following concussive brain injury in rats. J Cerebral Blood Flow Metabalism. 10:631-637.
Katayama, Y., Becker, D. P., Tamura, T., and Hovda, D. A. 1990. Massive increases in extracellular potassium and indiscriminate release of glutamate following concussive brain injury. J. Neurosurg. 73:889-900.
Hayes, R. L., Jenkins, L. W., Lyeth, B. G. et al. 1988. Pretreatment with phencyclidine, an N-methyl-D-aspartate antagonist, attenuates long-term behavioral deficits in the rat produced by traumatic brain injury. J. Neurotrauma. 5:259-274.
Hayes, R. L., Jenkins, L. W., and Lyeth, B. G. 1992. Neuropharmacological mechanisms of traumatic brain injury: Acetylcholine and excitatory amino acids. J. Neurotrauma suppl. 1:173-187.
McIntosh, T. K. 1993. Novel pharmacological therapies in the treatment of experimental traumatic brain injury: A review. J Neurotrauma. 10:215-61.
Robinson, S. E., Borrelli, G. S., Ang, J. L., Pascua, J. R., McDowell, K. P., and Enters, E. K. 1990. The effect of acetylcholine depletion on behavior following traumatic brain injury. Brain Res. 509:41-46.
Gorman, L. K., Fu, K., Hovda, D. A., Becker, D. P., and Katayama, Y. 1989. Analysis of acetylcholine release following concussive brain injury in the rat. (Abstract) J. Neurotrauma. 6:203.
Siesjoe, B. K., and Bengtsson, F. 1989. Calcium fluxes, calcium antagonists, and calcium related pathology in brain ischemia, hypoglycemia, and spreading depression a unifying hypothesis. J. Cereb. Blood Flow Metab. 9:127-140.
Fineman, I., Hovda, D., Smith, M., Yoshino, A., and Becker, D. 1993. Concussive brain injury is associated with a prolonged accumulation of calcium: a 45Ca autoradiographic study. Brain Research 624:94-102.
Kampfl, A., Posmantur, R., Nixon, R., Grynspan, F., Zhao, X., Liu, S. J., Newcomb, J., Clifton, G. L., Hayes, R. 1996. Calpain 1 activation and calpain 1 mediated cytoskeletal proteolysis following traumatic brain injury. J. Neurochemistry 67:1575-1583.
Kampfl, A., Posmantur, R., Clifton, G. L., and Hayes, R. L. 1997. Therapeutic implications of calpain inhibitors in traumatic brain injury. J. Neurotrauma 14:121-134.
Crumrine, R. C., Dubyak, G., and Lamanna, J. C. 1990. Decreased protein kinase C activity during cerebral ischemia and after reperfusion in the adult rat. J. Neurochem. 55:2001-2007.
Weiloch, T., Cardell, M., Bingren, H., Zivin, J., and Saitoh, K. 1991. Changes in the activity of protein kinase c and the differential subcellular redistribution of its isoenzymes in the rat striatum during and following transient forebrain ischemia. J. Neurochem. 56:1227-1233.
Hanson, S. K., Grotta, J. C., Waxham, M. N., Aronowski, J., and Ostrow, C. 1994. Calcium/calmodulin-dependent protein kinase II activity in focal ischemia with reperfusion in rats. Stroke. 25:466-473.
Yang, K. Y., Taft, W. C., Dixon, C. E., Yu, R. K., and Hayes, R. L. 1994. Endogenous phosphorylation of a 61000 dalton hippocampal protein increases following TBI. J. Neurotrauma. 11:523-532.
Dash, P. K., Moore, A. N., and Dixon, C. E. 1994. Spatial memory deficits, increased phosphorylation of the transcription factor creb and induction of the AP-1 complex following experimental brain injury. J. Neuroscience. 15:2030-2039.
Yamasaki, Y., Onodera, H., Adachi, K., Shozuhara, H., and Kogure, K. 1992. Alteration in the immunoreactivity of the calcineurin subunits after ischemia hippocampal damage. Neuroscience. 49:545-56.
Morioka, M., Fukunaga, K., Yasugawa, S., Nagahiro, S., Ushio, Y., and Miyamoto, T. 1992. Regional and temporal alterations in Ca2+/calmodulin-dependent protein kinase II and calcineurin in the hippocampus of rat brain after transient forebrain ischemia. J. Neurochem. 58:1798-809.
Siman, R. and Noszek, J. C. 1988. Excitatory amino acids activate calpain 1 and induce structural protein breakdown in vivo. Neuron. 1:279-287.
Melloni, E., and Pontremoli, S. 1989. The calpains. Trends Neurosci. 12:438-444.
Saido, T. C., Shibata, M., Takenawa, T., Murofushi, H., and Suzuki, K. 1992. Positive regulation of mu-calpin action by polyphosphoinositides. J. Biol. Chem. 267:24585-90.
Wang, K. W. and Yuen, P. W. 1994. Calpain inhibition: an overview of its therapeutic potential. Trends. Pharmacol. Sci. 15:412-419.
Bartus, R. T., Hayward, N. L., Elliott, P. J., Sawyer, S. D., Baker, K. L., Dean, R. L., Akyama, B. S., Straub, J. A., Harbeson, S. L., Li, Z., and Powers, J. 1994. Calpain inhibitor AK295 protects neurons from focal brain ischemia. Effects of post occlusion intra-arterial administration. Stroke. 25:2265-2270.
Dautingy, A., Pham-Dinh, D., Roussel, C., Felix, J. M., Nussbaum, J. L., and Jolles, T. 1988. The large neurofilament subunit (NF-H) of the rat: cDNA cloning and in situ detection. Biochem Biophys. Res. Commun. 154:1099-1106.
Julien, J. P. and Mushynski, W. E. 1982. Multiple phosphorylation sites in mammalian neurofilament polypeptides. J. Biol. Chem. 257:10467-10470.
Nixon, R. A. and Sihag, R. K. 1991. Neurofilament phosphorylation: a new look at regulation and function. T.I.N.S. 14:501-506.
Kamakura, K., Ishiura, S., Susuki, K., Sugita, H., and Takaku, F. 1985. Calcium-activated neutral protease in the peripheral nerve, which requires uM order Ca2+, and its effect on the neurofilament triplet. J. Neurosci. Res. 13:391-403.
Dixon, C. E., Clifton, G. L., Lighthall, J. W., Yaghmai, A. A., and Hayes, R. L. 1991. A controlled cortical impact model of traumatic brain injury in the rat. J. Neurosci. Meth. 39:253-262.
Hamm, R. J., Dixon, C. E., Gbadebo, D. M., Singha, A. K., Jenkins, L. W., Lyeth, B. G., and Hayes, R. L. 1992. Cognitive deficits following traumatic brain injury produced by controlled cortical impact. J. Neurotrauma. 9:11-20.
Goodman, J. C., Cherian, L., Bryan, R. M., and Robertson, CS 1994. Lateral cortical impact injury in rats: Pathological effects of varying cortical compression and impact velocity. J. Neurotrauma. 11:587-597.
Sutton, R. L., Lescaudron, L., and Stein, D.G. 1993. Unilateral cortical contusion injury in the rat: Vascular diruption and temporal development of cortical necrosis. J. Neurotrauma. 10:135-149.
Dixon, C. F., Lyeth, B. G., Giebel, M. L., Hamm, R. J., Povlishock, J. P., Becker, D. P., and Hayes, R. L. 1987. A fluid percussion model of experimental brain injury in the rat. J. Neurosurg. 67:110-19.
Towbin, H., Staehelin, T., and Gordon, J. 1979. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. USA. 76:4350-4354.
Lee, V. M. Y., Carden, M. J., Schlaepfer, W. W., and Trojanowski, J. Q. 1987. Monoclonal antibodies distinguish several differentially phosphorylated states of the two largest rat neurofilament subunits (NF-H and NF-M) and demonstrate their existence in the normal nervous system of adult rats. J. Neurosci. 7:3474-3488.
Harris, A. S., Croall, D. E., and Morrow, J. S. 1988. Calmodulin regulates fodrin susceptibility to cleavage by calcium-dependent protease I. J. Biol. Chem. 263:17401-17408.
Stabach, P. R., Cianci, C. D., Glantz, S. B., Zhang, Z., and Morrow, J. S. 1997. Site-directed mutagenesis of alpha II spectrin at codon 1175 modulates its mu-calpain susceptibility. Biochemistry 36:57-65.
Seubert, P., Baudry, M., Dudek, S., and Lynch G. 1987. Calmodulin stimulates the degradation of brain spectrin by calpain. Synapse 1(1):20-24.
Harris, A. S., Croall, D. E., Morrow, J. S. 1988. The calmodulin-binding site in alpha-fodrin is near the calcium-dependent protease-I cleavage site. J. Bio.l Chem. 30:15754-15761.
Nicholson, D. W., Ambereen, A. N., Thornberry, N. A., Vallancourt, J. P. et al. 1995 Identification and inhibition of the ICE/CED-3 protease necessary for mammalian apoptosis. Nature. 376:37-43.
Zhao, X., Newcomb, J. K., Posmantur, R., Wang, K. K. W., Pike, B. R., Hayes, R. L. 1997. pH dependency of μ-calpain and m-calpain activity assayed by casein zymography following traumatic brain injury in the rat. Neurosci Lett. (Submitted).
Chin, T. K., Eagles, P. A., and Maggs, A. 1983. The proteolytic digestion of ox neurofilament with trypsin and α-chymotrypsin. Biochem. J. 215:239-252.
Nixon, R. A. and Marotta, C. A. 1984. Degradation of neurofilament proteins by purified human brain cathepsin D. J. Neurochem. 43:507-516.
Susuki, H., Takeda, M., Nakamura, Y., Kato, Y., Tada, K., Hariguchi, S., and Nishimura, T. 1984. Neurofilament degradation by bovine brain cathepsin D. Neurosci. Lett. 89:240-245.
Martin, S. J., O'Brien, G. A., Nishioka, W. K., McGahon, A. J., Mahboubia, T., Saido, T. C., and Green, D. 1995. Proteolysis of fodrin during apoptosis. J. Biol. Chem. 270:6425-6428.
Wang, K. K., Nath, R., Posner, A., Raser, K. J., Buroker-Kilgore, M., Hajimohammadreza I, et al. 1996. An alpha-mercaptoacrylic acid derivative is a selective nonpeptide cell-permeable calpain inhibitor and is neuroprotective. Proc. Natl. Acad. Sci. 93:6687-6692.
Siman, R., Noszek, J. C., and Kegerise, C. 1989. Calpain I activation is specifically related to excitatory amino acid induction of hippocampal damage. J. Neuroscience. 9:1579-1590.
Roberts-Lewis, J. M., Savage, M. J., Marcy, V. R., Pinsker, L. R., and Siman, R. 1994. Immunolocalization of calpain 1 mediated spectrin degradation to vulnerable neurons in ischemic gerbil brain. J. Neurosci. 14:3934-3944.
Goto, K., Iwamoto, T., and Kndo, H. 1994. Localization of mRNA for calpain and calpastatin in adult rat brain by in situ hybridization histochemistry. Molecular Brain Research 23:40-46.
Kawashima, S., Hayashi, M., Saito, Y., Kasai, Y, Y., and Imahori, K. 1988. Tissue distribution of calcium-activated neutral proteinases in rat. Biochim. Biophys. Acta. 1088:130-135.
Kubota, S., Onaka, T., Murofushi, H., Ohsawa, N., and Takaku, F. 1986. Purification and characterization of high Ca2+ requiring neutral protease from porcine and bovine brain. Biochemistry, 25:8396-8402.
Li, J., Grynspan, F., Berman, S., Nixon, R., Bursztajn, S. 1996. Regional differences in gene expression for calcium activated neutral proteases (calpains) and their endogenous inhibitor calpastatin in mouse brain and spinal cord. J. Neurobiol., 30(2):177-191.
Pant, H. C. 1988. Dephosphorylation of neurofilament proteins enhances their susceptibility to degradation by calpain. Biochem. J. 256:665-668.
Nixon, R. A. 1993. The regulation of neurofilament protein dynamics by phosphorylation: clues to neurofibrillary pathobiology. Brain Pathol. 3:29-38.
Greenwood, J. A., Troncoso, J. C., Costello, A. C., and Johnson, G. C. 1993. Phosphorylation modulates calpain-mediated proteolysis and calmodulin binding of the 200-kDa and 160 kDa neurofilament proteins. J. Neurochem. 61:191-199.
Litersky, J. M., Scott, C. W., and Johnson, G. V. 1993. Phosphorylation, calpain proteolysis and tubulin binding of recombinant human tau isoforms. Brain Res. 604:32-40.
Litersky, J. M., and Johnson, G. V. 1995. Phosphorylation of tau in situ: Inhibition of calcium-dependent proteolysis. J. Neurochemistry 65:903-11.
Pettus, E. H., Christman, C. W., Giebel, M. L., and Povlishock, J. T. 1994. Traumatically induced altered membrane permeability: Its relationship to traumatically induced reactive axonal change. J. Neurotrauma 11:507-521.
Soussan, L., Tchernakov, K., Bachar-Lavar, O., Yuvan, T., Wertman, E., and Michaelson, D. M. 1994. Antibodies to different isoforms of the heavy neurofilament protein (NF-H) in normal aging and Alzheimers disease. Molecular Neurobiology 9:83-91.
Nixon, R. A., Saito, K. I., Grynspan, F., Griffin, W. R. Katayama, S., Honda, T., Mohan, P. S., Shea, T. B., and Beermann, M. 1994. Calcium-activated neutral proteinase (calpain) system in aging and Alzheimers disease. Annals of the New York Academy of Sciences. 747:77-91.
Smith, M. A., Rudnicka-Nawrot, M., Richey, P. L., Praprotnil, D., Mulvihill, P., Miller, C. A., and Sayre, G. 1995. Carbonyl-related posttranslational modification of neurofilament protein in the neurofibrillary pathology of Alzheimer's disease. J. Neurochemistry. 64:2660-2666.
Nagaraja, T. N., Gourie-Devi, M., Nalini, A., and Raju, T. R. 1994. Neurofilament phosphorylation is enhanced in cultured chick spinal neurons exposed to cerebrospinal fluid from amyotrophic lateral sclerosis patients. Acta Neuropathologica 88:349-52.
Migheli, A., Attanasio, A., and Schiffer, D. 1994. Ubiquitin and neurofilament expression in anterior horn cells in amytrophic lateral sclerosis: possible clues to the pathogenesis. Neuropathology and Applied Neurobiology. 20:282-289.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Posmantur, R.M., Zhao, X., Kampfl, A. et al. Immunoblot Analyses of the Relative Contributions of Cysteine and Aspartic Proteases to Neurofilament Breakdown Products Following Experimental Brain Injury in Rats. Neurochem Res 23, 1265–1276 (1998). https://doi.org/10.1023/A:1020792132629
Issue Date:
DOI: https://doi.org/10.1023/A:1020792132629