Abstract
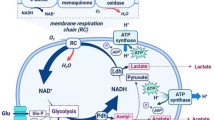
Lactic acid bacteria (LAB) constitute a heterogeneous group of bacteria that are traditionally used to produce fermented foods. The industrialization of food bio-transformations increased the economical importance of LAB, as they play a crucial role in the development of the organoleptique and hygienic quality of fermented products. Therefore, the reliability of starter strains in terms of quality and functional properties (important for the development of aroma and texture), but also in terms of growth performance and robustness has become essential. These strains should resist to adverse conditions encountered in industrial processes, for example during starter handling and storage (freeze-drying, freezing or spray-drying). The development of new applications such as life vaccines and probiotic foods reinforces the need for robust LAB since they may have to survive in the digestive tract, resist the intestinal flora, maybe colonize the digestive or uro-genital mucosa and express specific functions under conditions that are unfavorable to growth (for example, during stationary phase or storage). Also in nature, the ability to quickly respond to stress is essential for survival and it is now well established that LAB, like other bacteria, evolved defense mechanisms against stress that allow them to withstand harsh conditions and sudden environmental changes. While genes implicated in stress responses are numerous, in LAB the levels of characterization of their actual role and regulation differ widely between species. The functional conservation of several stress proteins (for example, HS proteins, Csp, etc) and of some of their regulators (for example, HrcA, CtsR) renders even more striking the differences that exist between LAB and the classical model micro-organisms. Among the differences observed between LAB species and B. subtilis, one of the most striking is the absence of a σB orthologue in L. lactis ssp. lactisas well as in at least two streptococci and probably E. faecalis. The overview of LAB stress responses also reveals common aspects of stress responses. As in other bacteria, adaptive responses appear to be a usual mode of stress protection in LAB. However, the cross-protection to other stress often induced by the expression of a given adaptive response, appears to vary between species. This observation suggests that the molecular bases of adaptive responses are, at least in part, species (or even subspecies) specific. A better understanding of the mechanisms of stress resistance should allow to understand the bases of the adaptive responses and cross protection, and to rationalize their exploitation to prepare LAB to industrial processes. Moreover, the identification of crucial stress related genes will reveal targets i) for specific manipulation (to promote or limit growth) , ii) to develop tools to screen for tolerant or sensitive strains and iii) to evaluate the fitness and level of adaptation of a culture. In this context, future genome and transcriptome analyses will undoubtedly complement the proteome and genetic information available today, and shed a new light on the perception of, and the response to, stress by lactic acid bacteria.
Similar content being viewed by others
References
Abdelal A T (1979) Arginine catabolism by microorganisms. Annu Rev Microbiol. 33: 139–168.
Abe K, Hayashi H& Maloney P C (1996) Exchange of aspartate and alanine: machanism for development of a proton-motive force in bacteria. J. Biol. Chem. 271: 3079–3084.
Adamowicz M, Kelley P M& Nickerson K W (1991) Detergent (sodium dodecyl sulfate) shock proteins in Escherichia coli. J. Bacteriol. 173: 229–233.
Archibald F S& Fridovich I (1981) Manganese, superoxide dismutase, and oxygen tolerance in some lactic acid bacteria. J Bacteriol. 146: 928–936.
Arena M E, Saguir F M& Manca de Nadra M C (1999) Arginine, citrulline and ornithine metabolism by lactic acid bacteria from wine. Int. J. Food Microbiol. 52: 155–161.
Arikado E, Ishihara H, Ehara T, Shibata C, Saito H, Kakegawa T, Igarashi K& Kobayashi H (1999) Enzyme level of enterococcal F1Fo-ATPase is regulated by pH at the step of assembly. Eur. J. Biochem. 259: 262–268.
Arnau J, Sorensen K I, Appel K F, Vogensen F K& Hammer K (1996) Analysis of heat shock gene expression in Lactococcus lactis MG1363. Microbiology 142: 1685–1691.
Auffray Y, Gansel X, Thammavongs B& Boutibonnes P (1992) Heat-shock induced protein synthesis in Lactococcus lactis subsp. lactis. Curr. Microbiol. 24: 281–284.
Baati L, Fabre-Gea C, Auriol D& Blanc P J (2000) Study of the cryotolerance of Lactobacillus acidophilus: effect of culture and freezing conditions on the viability and cellular protein levels. Int. J. Food Microbiol. 59: 241–247.
Bae W, Jones P G& Inouye M (1997) CspA, the major cold shock protein of Escherichia coli, negatively regulates its own gene expression. J. Bacteriol. 179: 7081–7088.
Bae W, Xia B, Inouye M& Severinov K (2000) Escherichia coli CspA-family RNA chaperones are transcription antiterminators. Proc. Natl. Acad. Sci. U.S.A. 97: 7784–7789.
Baev D, England R& Kuramitsu H K (1999) Stress-induced membrane association of the Streptococcus mutans GTP-binding protein, an essential G protein, and investigation of its physiological role by utilizing an antisense RNA strategy. Infect Immun. 67: 4510–4516.
Bakker E P& Harold F M (1980) Energy coupling to potassium transport in Streptococcus faecalis. J. Biol. Chem. 255: 433–440.
Becker G, Klauck E& Hengge-Aronis R (1999) Regulation of RpoS proteolysis in Escherichia coli: the response regulator RssB is a recognition factor that interacts with the turnover element in RpoS. Proc. Natl. Acad. Sci. U.S.A. 96: 6439–6444.
Belli W A & Marquis R E (1991) Adapation of Strepococcus mutans and Enteroccus hirae to acid stress in continuous culture. Appl. Environ. Microbiol. 57: 1134–1138.
Bernhardt J, Volker U, Volker A, Antelmann H, Schmid R, Mach H& Hecker M (1997) Specific and general stress proteins in Bacillus subtilis-a two-dimensional protein electrophoresis study. Microbiology 143: 999–1017.
Blank L M, Koebmann B J, Michelsen O, Nielsen L K& Jensen P R (2001) Hemin reconstitutes proton extrusion in an H(+)-ATPasenegative mutant of Lactococcus lactis. J. Bacteriol. 183: 6707–6709.
Bolhuis H, Molenaar D, Poelarends G, van Veen H W, Poolman B, Driessen A J& Konings W N (1994) Proton motive forcedriven and ATP-dependent drug extrusion systems in multidrugresistant Lactococcus lactis. J Bacteriol. 176: 6957–6964.
Bolotin A, Mauger S, Malarme K, Ehrlich S D& Sorokin A (1999) Low-redundancy sequencing of the entire Lactococcus lactis IL1403 genome. Antonie Van Leeuwenhoek 76: 27–76.
Bolotin A, Wincker P, Mauger S, Jaillon O, Malarme K, Weissenbach J, Ehrlich S D& Sorokin A (2001) The complete genome sequence of the lactic acid bacterium Lactococcus lactis ssp. lactis IL1403. Genome Res. 11: 1–23.
Bond D R& Russell J B (1996) A role for fructose 1,6-diphosphate in the ATPase-mediated energy spilling reaction of Streptococcus bovis. Appl. Environ. Microbiol. 62: 2095–2099.
Bouvier J, Bordes P, Romeo Y, Fourcans A, Bouvier I& Gutierrez C (2000) Characterization of OpuA, a glycine-betaine uptake system of Lactococcus lactis. J. Mol. Microbiol. Biotechnol. 2: 199–205.
Boyd D A, Cvitkovitch D G, Bleiweis A S, Kiriukhin M Y, Debabov D V, Neuhaus F C& Hamilton I R (2000) Defects in D-alanyllipoteichoic acid synthesis in Streptococcus mutans results in acid sensitivity. J. Bacteriol. 182: 6055–6065.
Brandi A, Pietroni P, Gualerzi C O& Pon C L (1996) Posttranscriptional regulation of CspA expression in Escherichia coli. Mol. Microbiol. 19: 231–240.
Breeuwer P, Drocourt J-L, Rombouts F M& Abee T (1996) A novel method for coninuous determination of the intracellular pH in bacteria with the internally conjugated fluorescent probe (and 6) carboxyfluorescein succinimidyl ester. Appl. Environ. Microbiol. 62: 178–183.
Broadbent J R& Lin C (1999) Effect of heat shock or cold shock treatment on the resistance of lactococcus lactis to freezing and lyophilization. Cryobiology 39: 88–102.
Broadbent J R, Oberg C J& Wei L (1998) Characterization of the Lactobacillus helveticus groESL operon. Res. Microbiol. 149: 247–253.
Caldon C E, Yoong P& March P E (2001) Evolution of a molecular switch: universal bacterial GTPases regulate ribosome function. Mol. Microbiol. 41: 289–297.
Cashel M, Gentry D, Hernandez V J& Vinella D (1996) The stringent response. In: Neidhardt F C, Ingraham J L, Low K B, Magasanik B, Schaechter M& Umberger H E (eds.), Escherichia coli and Salmonella typhimurium: Cellular and Molecular Biology (pp 1458–1496). American Society for Microbiology Press, Washington, DC.
Champomier-Verges M C, Zuniga M, Morel-Deville F, Perez-Martinez G, Zagorec M& Ehrlich S D (1999) Relationships between arginine degradation, pH and survival in Lactobacillus sakei. FEMS Microbiol. Lett. 180: 297–304.
Champomier-Verges M C, Chaillou S, Cornet M& Zagorec M (2001) Lactobacillus sakei: recent developments and future prospects. Res. Microbiol. 152: 839–848.
Champomier-Verges M C, Maguin E, Mistou M Y, Anglade P&Chich J F (2002) Lactic acid bacteria and proteomics: current knowledge and perspectives. J. Chromatogr. (in press).
Chang Y Y& Cronan Jr. J E, (1999) Membrane cyclopropane fatty acid content is a major factor in acid resistance of Escherichia coli. Mol. Microbiol. 33: 249–259.
Chapot-Chartier M P, Schouler C, Lepeuple A S, Gripon J C& Chopin M C (1997) Characterization of cspB, a cold-shockinducible gene from Lactococcus lactis, and evidence for a family of genes homologous to the Escherichia coli cspA major cold shock gene. J. Bacteriol. 179: 5589–5593.
Chastanet A, Prudhomme M, Claverys J P& Msadek T (2001) Regulation of Streptococcus pneumoniae clp genes and their role in competence development and stress survival. J. Bacteriol. 183: 7295–7307.
Chatterji D& Ojha A K (2001) Revisiting the stringent response, ppGpp and starvation signaling. Curr. Opin. Microbiol. 4: 160–165.
Chen Y Y& Burne R A (1996) Analysis of Streptococcus salivarius urease expression using continuous chemostat culture. FEMS Microbiol. Lett. 135: 223–229.
Chen Y Y, Weaver C A, Mendelsohn D R& Burne R A (1998) Transcriptional regulation of the Streptococcus salivarius 57.I urease operon. J. Bacteriol. 180: 5769–5775.
Condon S (1987) Responses of lactic acid bacteria to oxygen. FEMS Microbiol. Rev. 46: 269–280.
Chen Y Y, Weaver C A& Burne R A (2000) Dual functions of Streptococcus salivarius urease. J. Bacteriol. 182: 4667–4669.
Crosse A M, Greenway D L& England R R (2000) Accumulation of ppGpp and ppGp in Staphylococcus aureus 8325-4 following nutrient starvation. Lett. Appl. Microbiol. 31: 332–337.
Crow V L& Thomas T D (1982) Arginine metabolism in lactic Streptococci. J. Bacteriol. 150: 1024–1032.
Csonka L N& Hanson A D (1991) Prokaryotic osmoregulation: genetics and physiology. Annu. Rev. Microbiol. 45: 569–606.
Cunin R, Glansdorff N, Pierard A& Stalon V (1986) Biosynthesis and metabolism of arginine in bacteria. Microbiol. Rev. 50: 314–352.
Cvitkovitch D G, Gutierrez J A, Behari J, Youngman P J, Wetz J E, Crowley P F, Hillman J D, Brady L J& Bleiweis A S (2000) Tn917-lac mutagenesis of Streptococcus mutans to identify environmentally regulated genes. FEMS Microbiol. Lett. 182: 149–154.
De Angelis M, Bini L, Pallini V, Cocconcelli P S& Gobbetti M (2001) The acid-stress response in Lactobacillus sanfranciscensis CB1. Microbiology 147: 1863–1873.
de Urraza P& de Antoni G (1997) Induced cryotolerance of Lactobacillus delbrueckii subsp. bulgaricus LBB by preincubation at suboptimal temperature with a fermentable sugar. Cryobiology 35: 159–164.
Delcour J, Ferain T, Deghorain M, Palumbo E& Hols P (1999) The biosynthesis and functionality of the cell-wall of lactic acid bacteria. Antonie Van Leeuwenhoek 76: 159–184.
Delmas F, Pierre F, Coucheney F, Divies C& Guzzo J (2001) Biochemical and physiological studies of the small heat shock protein Lo18 from the lactic acid bacterium Oenococcus oeni. J. Mol. Microbiol. Biotechnol. 3: 601–610.
Derre I, Rapoport G& Msadek T (1999) CtsR, a novel regulator of stress and heat shock response, controls clp and molecular chaperone gene expression in gram-positive bacteria. Mol. Microbiol. 31: 117–131.
Derzelle S, Hallet B, Francis K P, Ferain T, Delcour J& Hols P (2000) Changes in cspL, cspP, and cspC mRNA abundance as a function of cold shock and growth phase in Lactobacillus plantarum. J. Bacteriol. 182: 5105–5113.
Diaz-Torres M L& Russell R R (2001) HtrA protease and processing of extracellular proteins of Streptococcus mutans. FEMS Microbiol. Lett. 204: 23–28.
Drici-Cachon Z, Guzzo J, Cavin J-F& Diviès C (1996) Acid tolerance in Leuconostoc oenos. Isolation and characterization of an acid-resistant mutant. Appl. Microbiol. Biotechnol. 44: 785–789.
Dunne C, Murphy L, Flynn S, O'Mahony L, O'Halloran S, Feeney M, Morrissey D, Thornton G, Fitzgerald G, Daly C, Kiely B, Quigley E M, O'Sullivan G C, Shanahan F& Collins J K (1999) Probiotics: from myth to reality. Demonstration of functionality in animal models of disease and in human clinical trials. Antonie Van Leeuwenhoek 76: 279–292.
Duwat P, Ehrlich S D& Gruss A (1995) The recA gene of Lactococcus lactis: characterization and involvement in oxidative and thermal stress. Mol. Microbiol. 17: 1121–1131.
Duwat P, Ehrlich S D& Gruss A (1999) Effects of metabolic flux on stress response pathways in Lactococcus lactis. Mol. Microbiol. 31: 845–858.
Duwat P, Sourice S, Cesselin B, Lamberet G, Vido K, Gaudu P, Le Loir Y, Violet F, Loubiere P& Gruss A (2001) Respiration capacity of the fermenting bacterium Lactococcus lactis and its positive effects on growth and survival. J. Bacteriol. 183: 4509–4516.
Earnshaw R G, Appleyard J& Hurst R M (1995) Understanding physical inactivation processes: combined preservation opportunities using heat, ultrasound and pressure. Int. J. Food Microbiol. 28: 197–219.
Eaton T, Shearman C& Gasson M (1993) Cloning and sequence analysis of the dnaK gene region of Lactococcus lactis subsp. lactis. J. Gen. Microbiol. 139: 3253–3264.
Elkins C A& Savage D C (1998) Identification of genes encoding conjugated bile salt hydrolase and transport in Lactobacillus johnsonii 100-100. J. Bacteriol. 180: 4344–4349.
Engesser D M& Hammes W P (1994) Non-heme catalase activity of lactic acid bacteria. Syst. Appl. Microbiol. 17: 11–19.
Eymann C, Mach H, Harwood C R& Hecker M (1996) Phosphate-starvation-inducible proteins in Bacillus subtilis: a two-dimensional gel electrophoresis study. Microbiology 142: 3163–3170.
Fabret C& Hoch J A (1998) A two-component signal transduction system essential for growth of Bacillus subtilis: implications for anti-infective therapy. J. Bacteriol. 180: 6375–6383.
Fang L, Jiang W, Bae W& Inouye M (1997) Promoter-independent cold-shock induction of cspA and its derepression at 37 degrees C by mRNA stabilization. Mol. Microbiol. 23: 355–364.
Fenoll A, Munoz R, Garcia E& de la Campa A G (1994) Molecular basis of the optochin-sensitive phenotype of pneumococcus: characterization of the genes encoding the F0 complex of the Streptococcus pneumoniae and Streptococcus oralis H(+)-ATPases. Mol. Microbiol. 12: 587–598.
Ferretti J. J, McShan W M, Ajdic D, Savic D J, Savic G, Lyon K, Primeaux C, Sezate S, Suvorov A N, Kenton S, Lai H S, Lin S P, Qian Y, Jia H G, Najar F Z, Ren Q, Zhu H, Song L, White J, Yuan X, Clifton S W, Roe B A& McLaughlin R (2001) Complete genome sequence of an M1 strain of Streptococcus pyogenes. Proc. Natl. Acad. Sci. U.S.A. 98: 4658–4663.
Fitzgerald J R& Musser J M (2001) Evolutionary genomics of pathogenic bacteria. Trends Microbiol. 9: 547–553.
Flahaut S, Frere J, Boutibonnes P& Auffray Y (1996a) Comparison of the bile salts and sodium dodecyl sulfate stress responses in Enterococcus faecalis. Appl. Environ. Microbiol. 62: 2416–2420.
Flahaut S, Hartke A, Giard J C, Benachour A, Boutibonnes P& Auffray Y (1996b) Relationship between stress response toward bile salts, acid and heat treatment in Enterococcus faecalis. FEMS Microbiol. Lett. 138: 49–54.
Foucaud-Sceunemann C&Poquet I (2002) The Lactococcus lactis HtrA protease is induced and essential for cell survival under stress conditions. In preparation.
Francis K P, Mayr R, von Stetten F, Stewart G S& Scherer S (1998) Discrimination of psychrotrophic and mesophilic strains of the Bacillus cereus group by PCR targeting of major cold shock protein genes. Appl. Environ. Microbiol. 64: 3525–3529.
Frees D& Ingmer H (1999) ClpP participates in the degradation of misfolded protein in Lactococcus lactis. Mol. Microbiol. 31: 79–87.
Frees D, Varmanen P& Ingmer H (2001) Inactivation of a gene that is highly conserved in Gram-positive bacteria stimulates degradation of non-native proteins and concomitantly increases stress tolerance in Lactococcus lactis. Mol. Microbiol. 41: 93–103.
Futai M, Noumi T& Maeda M (1989) ATP synthase (H+-ATPase): results by combined biochemical and molecular biological approaches. Annu. Rev. Biochem. 58: 111–136.
Galperin M Y, Walker D R& Koonin E V (1998) Analogous enzymes: independent inventions in enzyme evolution. Genome Res. 8: 779–790.
Garcia-Quintans N, Magni C, de Mendoza D& Lopez P (1998) The citrate transport system of Lactococcus lactis subsp. lactis biovar diacetylactis is induced by acid stress. Appl. Environ. Microbiol. 64: 850–857.
Giard J C, Hartke A, Flahaut S, Benachour A, Boutibonnes P& Auffray Y (1996) Starvation-induced multiresistance in Enterococcus faecalis JH2-2. Curr. Microbiol. 32: 264–271.
Giard J C, Hartke A, Flahaut S, Boutibonnes P& Auffray Y (1997) Glucose starvation response in Enterococcus faecalis JH2-2: survival and protein analysis. Res. Microbiol. 148: 27–35.
Giard J C, Rince A, Capiaux H, Auffray Y& Hartke A (2000) Inactivation of the stress-and starvation-inducible gls24 operon has a pleiotrophic effect on cell morphology, stress sensitivity, and gene expression in Enterococcus faecalis. J. Bacteriol. 182: 4512–4520.
Giard J C, Laplace J M, Rince A, Pichereau V, Benachour A, Leboeuf C, Flahaut S, Auffray Y& Hartke A (2001) The stress proteome of Enterococcus faecalis. Electrophoresis 22: 2947–2954.
Glaasker E, Konings W N& Poolman B (1996a) Glycine betaine fluxes in Lactobacillus plantarum during osmostasis and hyperand hypo-osmotic shock. J. Biol. Chem. 271: 10060–10065.
Glaasker E, Konings W N& Poolman B (1996b) Osmotic regulation of intracellular solute pools in Lactobacillus plantarum. J. Bacteriol. 178: 575–582.
Glaasker E, Heuberger E H, Konings W N& Poolman B (1998a) Mechanism of osmotic activation of the quaternary ammonium compound transporter (QacT) of Lactobacillus plantarum. J Bacteriol. 180: 5540–5546.
Glaasker E, Tjan F S, Ter Steeg P F, Konings W N& Poolman B (1998b) Physiological response of Lactobacillus plantarum to salt and nonelectrolyte stress. J. Bacteriol. 180: 4718–4723.
Goldenberg D, Azar I& Oppenheim A B (1996) Differential mRNA stability of the cspA gene in the cold-shock response of Escherichia coli. Mol. Microbiol. 19: 241–248.
Gordia S& Gutierrez C (1996) Growth-phase-dependent expression of the osmotically inducible gene osmC of Escherichia coli K-12. Mol. Microbiol. 19: 729–736.
Gostick D O, Griffin H G, Shearman C A, Scott C, Green J, Gasson M J& Guest J R (1999) Two operons that encode FNR-like proteins in Lactococcus lactis. Mol. Microbiol. 31: 1523–1535.
Gouesbet G, Jan G& Boyaval P (2002) Two-dimensional electrophoresis study of Lactobacillus delbrueckii subsp. bulgaricus thermotolerance. Appl. Environ. Microbiol. 68: 1055–1063.
Graumann P, Schroder K, Schmid R& Marahiel M A (1996) Cold shock stress-induced proteins in Bacillus subtilis. J. Bacteriol. 178: 4611–4619.
Graumann P, Wendrich T M, Weber M H, Schroder K& Marahiel M A (1997) A family of cold shock proteins in Bacillus subtilis is essential for cellular growth and for efficient protein synthesis at optimal and low temperatures. Mol. Microbiol. 25: 741–756.
Grkovic S, Brown M H& Skurray R A (2001) Transcriptional regulation of multidrug efflux pumps in bacteria. Semin. Cell Dev. Biol. 12: 225–237.
Guedon E, Serror P, Ehrlich S D, Renault P& Delorme C (2001) Pleiotropic transcriptional repressor CodY senses the intracellular pool of branched-chain amino acids in Lactococcus lactis. Mol. Microbiol. 40: 1227–1239.
Guerzoni M E, Lanciotti R& Cocconcelli P S (2001) Alteration in cellular fatty acid composition as a response to salt, acid, oxidative and thermal stresses in Lactobacillus helveticus. Microbiology 147: 2255-2264.
Guillot A, Obis D& Mistou M Y (2000) Fatty acid membrane composition and activation of glycine-betaine transport in Lactococcus lactis subjected to osmotic stress. Int. J. Food Microbiol. 55: 47–51.
Gunn J S (2000) Mechanisms of bacterial resistance and response to bile. Microbes Infect. 2: 907–913.
Gutierrez J A, Crowley P J, Brown D P, Hillman J D, Youngman P& Bleiweis A S (1996) Insertional mutagenesis and recovery of interrupted genes of Streptococcus mutans by using transposon Tn917: preliminary characterization of mutants displaying acid sensitivity and nutritional requirements. J. Bacteriol. 178: 4166–4175.
Gutierrez J A, Crowley P J, Cvitkovitch D G, Brady L J, Hamilton I R, Hillman J D& Bleiweis A S (1999) Streptococcus mutans ffh, a gene encoding a homologue of the 54 kDa subunit of the signal recognition particle, is involved in resistance to acid stress. Microbiology 145: 357–366.
Guzzo J, Cavin J& Divies C (1994) Induction of stress proteins in Leuconostoc oenos to perform direct inoculation of wine. Biotechnol. Lett. 16: 1189–1194.
Guzzo J, Delmas F, Pierre F, Jobin M P, Samyn B, Van Beeumen J, Cavin J F& Divies C (1997) A small heat shock protein from Leuconostoc oenos induced by multiple stresses and during stationary growth phase. Lett. Appl. Microbiol. 24: 393–396.
Guzzo J, Jobin M-P, Delmas F, Fortier L-C, Garmyn D, Tourdot-Maréchal R, Lee B& Diviès C (2000) Regulation of stress response in Oenococcus oeni as a function of environmental changes and growth phase. Int. J. Food Microbiol. 55: 27–31.
Hahn K, Faustoferri R C& Quivey Jr. R G (1999) Induction of an AP endonuclease activity in Streptococcus mutans during growth at low pH. Mol. Microbiol. 31: 1489–1498.
Hanna M M& Liu K (1998) Nascent RNA in transcription complexes interacts with CspE, a small protein in E. coli implicated in chromatin condensation. J. Mol. Biol. 282: 227–239.
Hanna M N, Ferguson R J, Li Y H& Cvitkovitch D G (2001) uvrA is an acid-inducible gene involved in the adaptive response to low pH in Streptococcus mutans. J. Bacteriol. 183: 5964–5973.
Hansen M C, Nielsen A K, Molin S, Hammer K, Kilstrup M, Palmer Jr. R J, Udsen C& White D C (2001) Changes in rRNA levels during stress invalidates results from mRNA blotting: fluorescence in situ rRNA hybridization permits renormalization for estimation of cellular mRNA levels. J. Bacteriol. 183: 4747–4751.
Harper D S& Loesche W J (1984) Growth and acid tolerance of human dental plaque bacteria. Arch. Oral Biol. 29: 843–848.
Hartke A, Bouché S, Gansel X, Boutibonnes P& Auffray Y (1994) Starvation-induced stress resistance in Lactococcus lactis subsp. lactis IL1403. Appl. Environ. Microbiol. 60: 3474–3478.
Hartke A, Bouche S, Laplace J-M, Benachour A, Boutibonnes P& Auffray Y (1995) UV-inducible proteins and UV-induced crossprotection against acid, ethanol, H2O2 or heat treatments in Lactococcus lactis subsp. lactis. Arch. Microbiol. 163: 329–336.
Hartke A, Bouché S, Giard J C, Benachour A, Boutibonnes P& Auffray Y (1996) The lactic acid stress response of Lactococcus lactis subsp. lactis. Curr. Microbiol. 33: 194–199.
Hartke A, Frere J, Boutibonnes P& Auffray Y (1997) Differential induction of the chaperonin GroEL and the Co-chaperonin GroES by heat, acid, and UV-irradiation in Lactococcus lactis subsp. lactis. Curr. Microbiol. 34: 23–26.
Hartke A, Giard J C, Laplace J M& Auffray Y (1998) Survival of Enterococcus faecalis in an oligotrophic microcosm: changes inmorphology, development of general stress resistance, and analysis of protein synthesis. Appl. Environ. Microbiol. 64: 4238–4245.
Hengge-Aronis R (1993) Survival of hunger and stress: the role of rpoS in early stationary phase gene regulation in E. coli. Cell 72: 165–168.
Hengge-Aronis R (1999) Interplay of global regulators and cell physiology in the general stress response of Escherichia coli. Curr. Opin. Microbiol. 2: 148–152.
Hertel C, Schmidt G, Fischer M, Oellers K& Hammes W P (1998) Oxygen-dependent regulation of the expression of the catalase gene katA of Lactobacillus sakei LTH677. Appl. Environ. Microbiol. 64: 1359–1365.
Higgins C F (1992) ABC transporters: from microorganisms toman. Annu. Rev. Cell Biol. 8: 67–113.
Higuchi T, Hayashi H& Abe K (1997) Exchange of glutamate and gamma-aminobutyrate in a Lactobacillus strain. J. Bacteriol. 179: 3362–3364.
Hoskins J, Alborn Jr. W E, Arnold J, Blaszczak L C, Burgett S, DeHoff B S, Estrem S T, Fritz L, Fu D J, Fuller W, Geringer C, Gilmour R, Glass J S, Khoja H, Kraft A R, Lagace R E, LeBlanc D J, Lee L N, Lefkowitz E J, Lu J, Matsushima P, McAhren S M, McHenney M, McLeaster K, Mundy C W, Nicas T I, Norris F H, O'Gara M, Peery R B, Robertson G T, Rockey P, Sun P M, Winkler M E, Yang Y, Young-Bellido M, Zhao G, Zook C A, Baltz R H, Jaskunas S R, Rosteck Jr. P R, Skatrud P L& Glass J I (2001) Genome of the bacterium Streptococcus pneumoniae strain R6. J. Bacteriol. 183: 5709–5717.
Hutkins R W& Nannen N L (1993) pH homeostasis in lactic acid bacteria. J. Dairy Sci. 76: 2354–2365.
Hutkins R W, Ellefson W L& Kashket E R (1987) Betaine transport imparts osmotolerance on a strain of Lactobacillus acidophilus. Appl. Environ. Microbiol. 53: 2275–2281.
Igarashi T, Kono Y& Tanaka K (1996) Molecular cloning of manganese catalase from Lactobacillus plantarum. J. Biol. Chem. 271: 29521–29524.
Ingmer H, Vogensen F K, Hammer K& Kilstrup M (1999) Disruption and analysis of the clpB, clpC, and clpE genes in Lactococcus lactis: ClpE, a new Clp family in gram-positive bacteria. J. Bacteriol. 181: 2075–2083.
Irvine A S& Guest J R (1993) Lactobacillus casei contains a member of the CRP-FNR family. Nucleic Acids Res. 21: 753.
Israelsen H, Madsen S M, Vrang A, Hansen E B& Johansen E (1995) Cloning and partial characterization of regulated promoters from Lactococcus lactis Tn917-lacZ integrants with the new promoter probe vector, pAK80. Appl. Environ. Microbiol. 61: 2540–2547.
Jayaraman G C, Penders J E& Burne R A (1997) Transcriptional analysis of the Streptococcus mutans hrcA, grpE and dnaK genes and regulation of expression in response to heat shock and environmental acidification. Mol. Microbiol. 25: 329–341.
Jensen N B, Melchiorsen C R, Jokumsen K V& Villadsen J (2001) Metabolic behavior of Lactococcus lactis MG1363 in microaerobic continuous cultivation at a low dilution rate. Appl. Environ. Microbiol. 67: 2677–2682.
Jiang W, Fang L& Inouye M (1996) The role of the 5'-end untranslated region of the mRNA for CspA, the major cold-shock protein of Escherichia coli, in cold-shock adaptation. J. Bacteriol. 178: 4919–4925.
Jiang W, Hou Y& Inouye M (1997) CspA, the major cold-shock protein of Escherichia coli, is an RNA chaperone. J. Biol. Chem. 272: 196–202.
Jobin M P, Garmyn D, Divies C& Guzzo J (1999) Expression of the Oenococcus oeni trxA gene is induced by hydrogen peroxide and heat shock. Microbiology 145: 1245-1251.
Kaan T, Jurgen B& Schweder T (1999) Regulation of the expression of the cold shock proteins CspB and CspC in Bacillus subtilis. Mol. Gen. Genet. 262: 351–354.
Kashket E R (1984) Bioenergetics of lactic acid bacteria: cytoplasmic pH and osmotolerance. FEMS Microbiol. Rev. 46: 233–244.
Kashket E R& Barker S L (1977) Effects of potassium ions on the electrical and pH gradients across the membrane of Streptococcus lactis cells. J. Bacteriol. 130: 1017–1023.
Kets E P W, Teunissen P J M& De Bont J A M (1996) Effect of compatible solutes on survival of lactic acid bacteria subjected to drying. Appl. Environ. Microbiol. 62: 259–261.
Kilstrup M, Jacobsen S, Hammer K& Vogensen F K (1997) Induction of heat shock proteins DnaK, GroEL, and GroES by salt stress in Lactococcus lactis. Appl. Environ. Microbiol. 63: 1826–1837.
Kim S G& Batt C A (1993) Cloning and sequencing of the Lactococcus lactis subsp. lactis groESL operon. Gene. 127: 121–126.
Kim W S& Dunn N W (1997) Identification of a cold shock gene in lactic acid bacteria and the effect of cold shock on cryotolerance. Curr. Microbiol. 35: 59–63.
Kim W S, Khunajakr N& Dunn N W (1998) Effect of cold shock on protein synthesis and on cryotolerance of cells frozen for long periods in Lactococcus lactis. Cryobiology 37: 86–91.
Kim W S, Ren J& Dunn N W (1999) Differentiation of Lactococcus lactis subspecies lactis and subspecies cremoris strains by their adaptive response to stresses. FEMS Microbiol. Lett. 171: 57–65.
Kim W S, Perl L, Park J H, Tandianus J E& Dunn N W (2001) Assessment of stress response of the probiotic Lactobacillus acidophilus. Curr. Microbiol. 43: 346–350.
Knauf H J, Vogel R F& Hammes W P (1992) Cloning, sequence, and phenotypic expression of katA, which encodes the catalase of Lactobacillus sake LTH677. Appl. Environ. Microbiol. 58: 832–839.
Kobayashi H, Murakami N& Unemoto T (1982) Regulation of the cytoplasmic pH in Streptococcus faecalis. J. Biol. Chem. 257: 13246–13252.
Koch B, Kilstrup M, Vogensen F K& Hammer K (1998) Induced levels of heat shock proteins in a dnaK mutant of Lactococcus lactis. J. Bacteriol. 180: 3873–3881.
Koebmann B J, Nilsson D, Kuipers O P& Jensen P R (2000) The membrane-bound H(+)-ATPase complex is essential for growth of Lactococcus lactis. J. Bacteriol. 182: 4738–4743.
Komatsu Y, Kaul S C, Iwahashi H& Obuchi K (1990) Do heat shock proteins provide protection against freezing? FEMS Microbiol. Lett. 60: 159–162.
Konings W N, Lolkema J S, Bolhuis H, van Veen H W, Poolman B& Driessen A J (1997) The role of transport processes in survival of lactic acid bacteria. Energy transduction and multidrug resistance. Antonie Van Leeuwenhoek. 71: 117–128.
Kono Y& Fridovich I (1983) Isolation and characterization of the pseudocatalase of Lactobacillus plantarum. J. Biol. Chem. 258: 6015–6019.
Koonin E V, Aravind L& Glaperin M Y (2000) A comparativegenomic view of the microbial stress response. In: Stortz G& Hengge-Aronis R (Eds.) Bacterial Stress Responses (pp 417–444). ASM Press, Washington, DC.
Kornberg A, Rao N N& Ault-Riche D (1999) Inorganic polyphosphate: a molecule of many functions. Annu. Rev. Biochem. 68: 89–125.
Kremer B H, van der Kraan M, Crowley P J, Hamilton I R, Brady L J& Bleiweis A S (2001) Characterization of the sat operon in Streptococcus mutans: evidence for a role of Ffh in acid tolerance. J. Bacteriol. 183: 2543–2552.
Kullen M J& Klaenhammer T R (1999) Identification of the pHinducible, proton-translocating F1F0-ATPase (atpBEFHAGDC) operon of Lactobacillus acidophilus by differential display: gene structure, cloning and characterization. Mol. Microbiol. 33: 1152–1161.
Kunji E R, Ubbink T, Matin A, Poolman B& Konings W N (1993) Physiological responses of Lactococcus lactis ML3 to alternating conditions of growth and starvation. Arch. Microbiol. 159: 372–379.
Kuroda A, Nomura K, Ohtomo R, Kato J, Ikeda T, Takiguchi N, Ohtake H& Kornberg A (2001) Role of inorganic polyphosphate in promoting ribosomal protein degradation by the Lon protease in E. coli. Science 293: 705–708.
Kvint K, Farewell A& Nystrom T (2000) RpoS-dependent promoters require guanosine tetraphosphate for induction even in the presence of high levels of sigma(s). J. Biol. Chem. 275: 14795–14798.
Lange R& Hengge-Aronis R (1991) Growth phase-regulated expression of bolA and morphology of stationary-phase Escherichia coli cells are controlled by the novel sigma factor sigma S. J. Bacteriol. 173: 4474–4481.
Laport M S, de Castro A C, Villardo A, Lemos J A, Bastos M C& Giambiagi-deMarval M (2001) Expression of the major heat shock proteins DnaK and GroEL in Streptococcus pyogenes: a comparison to Enterococcus faecalis and Staphylococcus aureus. Curr. Microbiol. 42: 264–268.
Lawrence J G, Hendrix R W& Casjens S (2001) Where are the pseudogenes in bacterial genomes? Trends Microbiol. 9: 535–540.
Lemos J A, Chen Y Y& Burne R A (2001) Genetic and physiologic analysis of the groE operon and role of the HrcA repressor in stress gene regulation and acid tolerance in Streptococcus mutans. J. Bacteriol. 183: 6074–6084.
Li Y H, Hanna M N, Svensater G, Ellen R P& Cvitkovitch D G (2001) Cell density modulates acid adaptation in Streptococcus mutans: implications for survival in biofilms. J. Bacteriol. 183: 6875–6884.
Lim E M, Ehrlich S D& Maguin E (2000) Identification of stress-inducible proteins in Lactobacillus delbrueckii subsp. bulgaricus. Electrophoresis 21: 2557–2561.
Lin M Y& Yen C L (1999) Antioxidative ability of lactic acid bacteria. J. Agric. Food Chem. 47: 1460–1466.
Lindahl T& Nyberg B (1972) Rate of depurination of native deoxyribonucleic acid. Biochemistry 11: 3610–3618.
Liu S, Asmundson R V, Gopal P K, Holland R& Crow V L (1998) Influence of reduced water activity on lactose metabolism by lactococcus lactis subsp. cremoris at different pH values. Appl. Environ. Microbiol. 64: 2111–2116.
Loewen P C, Hu B, Strutinsky J& Sparling R (1998) Regulation in the rpoS regulon of Escherichia coli. Can. J. Microbiol. 44: 707–717.
Lomovskaya O& Lewis K (1992) Emr, an Escherichia coli locus for multidrug resistance. Proc. Natl. Acad. Sci. U.S.A. 89: 8938–8942.
Lopez de Felipe F, Kleerebezem M, de Vos W M& Hugenholtz J (1998) Cofactor engineering: a novel approach to metabolic engineering in Lactococcus lactis by controlled expression of NADH oxidase. J. Bacteriol. 180: 3804–3808.
Lorca G L& de Valdez G F (1999) The effect of suboptimal growth temperature and growth phase on resistance of Lactobacillus acidophilus to environmental stress. Cryobiology 39: 144–149.
Lorca G L& Valdez G F (2001) A low-pH-inducible, stationaryphase acid tolerance response in Lactobacillus acidophilus CRL 639. Curr. Microbiol. 42: 21–25.
Ma Y& Marquis R E (1997) Thermophysiology of Streptococcus mutans and related lactic-acid bacteria. Antonie Van Leeuwenhoek 72: 91–100.
Ma D, Cook D N, Hearst J E& Nikaido H (1994) Efflux pumps and drug resistance in gram-negative bacteria. Trends Microbiol. 2: 489–493.
Ma Y, Curran T M& Marquis R E (1997) Rapid procedure for acid adaptation of oral lactic-acid bacteria and further characterization of the response. Can. J. Microbiol. 43: 143–148.
Mallonee D H& Hylemon P B (1996) Sequencing and expression of a gene encoding a bile acid transporter from Eubacterium sp. strain VPI 12708. J. Bacteriol. 178: 7053–7058.
Markham P N& Neyfakh A A (2001) Efflux-mediated drug resistance in Gram-positive bacteria. Curr. Opin. Microbiol. 4: 509–514.
Marquis R E, Bender G R, Murray D R& Wong A (1987) Arginine deiminase system and bacterial adaptation to acid environments. Appl. Environ. Microbiol. 53: 198–200.
Martin-Galiano A J, Ferrandiz M J& de la Campa A G (2001) The promoter of the operon encoding the F0F1 ATPase of Streptococcus pneumoniae is inducible by pH. Mol. Microbiol. 41: 1327–1338.
Martirani L, Raniello R, Naclerio G, Ricca E& De Felice M (2001) Identification of the DNA-binding protein, HrcA, of Streptococcus thermophilus. FEMS Microbiol. Lett. 198: 177–182.
Marty-Teysset C, Posthuma C, Lolkema J S, Schmitt P, Divies C& Konings W N (1996) Proton motive force generation by citrolactic fermentation in Leuconostoc mesenteroides. J. Bacteriol. 178: 2178–2185.
Marty-Teysset C, de la Torre F& Garel J (2000) Increased production of hydrogen peroxide by Lactobacillus delbrueckii subsp. bulgaricus upon aeration: involvement of an NADH oxidase in oxidative stress. Appl. Environ. Microbiol. 66: 262–267.
Mascarenhas J, Weber M H& Graumann P L (2001) Specific polar localization of ribosomes in Bacillus subtilis depends on active transcription. EMBO Rep. 2: 685–689.
Mayo B, Derzelle S, Fernandez M, Leonard C, Ferain T, Hols P, Suarez J E& Delcour J (1997) Cloning and characterization of cspL and cspP, two cold-inducible genes from Lactobacillus plantarum. J. Bacteriol. 179: 3039–3042.
Mechold U, Cashel M, Steiner K, Gentry D& Malke H (1996) Functional analysis of a relA/spoT gene homolog from Streptococcus equisimilis. J. Bacteriol. 178: 1401–1411.
Mercenier A, Muller-Alouf H& Grangette C (2000) Lactic acid bacteria as live vaccines. Curr. Issues Mol. Biol. 2: 17–25.
Miyoshi A, Gratadoux J J, Azevedo V, Rochat T, Duwat P, Sourice S, Oliveira S C, Gruss A&Langella P (2002) Expression of heterologous catalases confers high-level resistance to oxidative stress in Lactococcus lactis. In preparation.
Mogk A, Homuth G, Scholz C, Kim L, Schmid F X& Schumann W(1997) The GroE chaperonin machine is a major modulator of the CIRCE heat shock regulon of Bacillus subtilis. EMBO J. 16: 4579–4590.
Molenaar D, Bosscher J S, ten Brink B, Driessen A J& Konings W N (1993) Generation of a proton motive force by histidine decarboxylation and electrogenic histidine/histamine antiport in Lactobacillus buchneri. J. Bacteriol. 175: 2864–2870.
Moser S A& Savage D C (2001) Bile salt hydrolase activity and resistance to toxicity of conjugated bile salts are unrelated properties in lactobacilli. Appl. Environ. Microbiol. 67: 3476–3480.
Nannen N L& Hutkins R W (1991) Proton translocating adenosine triphosphatase activity in lacic acid bacteria. J. Dairy Sci. 74: 747–751.
Nilsson D, Lauridsen A A, Tomoyasu T& Ogura T (1994) A Lactococcus lactis gene encodes a membrane protein with putative ATPase activity that is homologous to the essential Escherichia coli ftsH gene product. Microbiology 140: 2601–2610.
Nishino K& Yamaguchi A (2001) Analysis of a complete library of putative drug transporter genes in Escherichia coli. J. Bacteriol. 183: 5803–5812.
Obis D, Guillot A, Gripon J C, Renault P, Bolotin A& Mistou M Y (1999) Genetic and biochemical characterization of a high-affinity betaine uptake system (BusA) in Lactococcus lactis reveals a new functional organization within bacterial ABC transporters. J. Bacteriol. 181: 6238–6246.
O'Connell-Motherway M, van Sinderen D, Morel-Deville F, Fitzgerald G F, Ehrlich S D& Morel P (2000) Six putative twocomponent regulatory systems isolated from Lactococcus lactis subsp. cremoris MG1363. Microbiology 146: 935–947.
Olsen E B, Russell J B& Henick-Kling T (1991) Electrogenic L-malate transport by Lactobacillus plantarum: a basis for energy derivation from malolactic fermentation. J. Bacteriol. 173: 6199–6206.
O'Sullivan E& Condon S (1999) Relationship between acid tolerance, cytoplasmic pH, and ATP and H+-ATPase levels in chemostat cultures of Lactococcus lactis. Appl. Environ. Microbiol. 65: 2287–2293.
Panoff J M, Legrand S, Thammavongs B& Boutibonnes P (1994) The cold shock response in Lactococcus subsp. lactis. Curr. Microbiol. 29: 213–216.
Panoff J M, Thammavongs B, Laplace J M, Hartke A, Boutibonnes P& Auffray Y (1995) Cryotolerance and cold adaptation in Lactococcus lactis subsp. lactis IL1403. Cryobiology 32: 516–520.
Panoff J M, Corroler D, Thammavongs B& Boutibonnes P (1997) Differentiation between cold shock proteins and cold acclimation proteins in a mesophilic gram-positive bacterium, Enterococcus faecalis JH2-2. J. Bacteriol. 179: 4451–4454.
Panoff J M, Thammavongs B& Gueguen M (2000) Cryoprotectants lead to phenotypic adaptation to freeze-thaw stress in Lactobacillus delbrueckii ssp. bulgaricus CIP 101027T. Cryobiology 40: 264–269.
Pebay M, Holl A C, Simonet J M& Decaris B (1995) Characterization of the gor gene of the lactic acid bacterium Streptococcus thermophilus CNRZ368. Res. Microbiol. 146: 371–383.
Perrin C, Guimont C, Bracquart P& Gaillard J L (1999) Expression of a new cold shock protein of 21.5 kDa and of the major cold shock protein by Streptococcus thermophilus after cold shock. Curr. Microbiol. 39: 342–347.
Persuh M, Turgay K, Mandic-Mulec I& Dubnau D (1999) The Nand C-terminal domains of MecA recognize different partners in the competence molecular switch. Mol. Microbiol. 33: 886–894.
Petersohn A, Brigulla M, Haas S, Hoheisel J D, Volker U& Hecker M (2001) Global analysis of the general stress response of Bacillus subtilis. J. Bacteriol. 183: 5617–5631.
Phadtare S& Inouye M(1999) Sequence-selective interactions with RNA by CspB, CspC and CspE, members of the CspA family of Escherichia coli. Mol. Microbiol. 33: 1004–1014.
Phadtare S, Yamanata K& Inouye M (2000) The cold-shock response. In: Stortz G& Hengge-Aronis R (Eds.) Bacterial Stress Response (pp 33–45). ASM Press, Washington, DC.
Poolman B, Nijssen R M& Konings W N (1987a) Dependence of Streptococcus lactis phosphate transport on internal phosphate concentration and internal pH. J. Bacteriol. 169: 5373–5378.
Poolman B, Smid E J, Veldkamp H& Konings W (1987b) Bioenergetic consequences of lactose starvation for continuously cultured Streptococcus cremoris and Streptococcus lactis. J. Bacteriol. 169: 1460–1468.
Poolman B, Molenaar D, Smid E J, Ubbink T, Abee T, Renault P P& Konings W N (1991) Malolactic fermentation: electrogenic malate uptake and malate/lactate antiport generate metabolic energy. J. Bacteriol. 173: 6030–6037.
Poolman B& Glaasker E (1998) Regulation of compatible solute accumulation in bacteria. Mol. Microbiol. 29: 397–407.
Poquet I, Saint V, Seznec E, Simoes N, Bolotin A& Gruss A (2000) HtrA is the unique surface housekeeping protease in Lactococcus lactis and is required for natural protein processing. Mol. Microbiol. 35: 1042–1051.
Presser K A, Ratkowsky D A& Ross T (1997) Modelling the growth rate of Escherichia coli as a function of pH and lactic acid concentration. Appl. Environ. Microbiol. 63: 2355–2360.
Price C W, Fawcett P, Ceremonie H, Su N, Murphy C K& Youngman P (2001) Genome-wide analysis of the general stress response in Bacillus subtilis. Mol. Microbiol. 41: 757–774.
Prieto-Alamo M J, Jurado J, Gallardo-Madueno R, Monje-Casas F, Holmgren A& Pueyo C (2000) Transcriptional regulation of glutaredoxin and thioredoxin pathways and related enzymes in response to oxidative stress. J. Biol. Chem. 275: 13398–13405.
Provenzano D& Klose K E (2000) Altered expression of the ToxR-regulated porins OmpU and OmpT diminishes Vibrio cholerae bile resistance, virulence factor expression, and intestinal colonization. Proc. Natl. Acad. Sci. U.S.A. 97: 10220–10224.
Putman M, van Veen H W& Konings W N (2000) Molecular properties of bacterial multidrug transporters. Microbiol. Mol. Biol. Rev. 64: 672–693.
Quivey Jr. R G, Faustoferri R C, Clancy K A& Marquis R E (1995) Acid adaptation in Streptococcus mutans UA159 alleviates sensitization to environmental stress due to RecA deficiency. FEMS Microbiol. Lett. 126: 257–261.
Quivey Jr. R G, Faustoferri R, Monahan K& Marquis R (2000a) Shifts in membrane fatty acid profiles associated with acid adaptation of Streptococcus mutans. FEMS Microbiol. Lett. 189: 89–92.
Quivey Jr. R G, Kuhnert W L& Hahn K (2000b) Adaptation of oral streptococci to low pH. Adv. Microb. Physiol. 42: 239–274.
Quivey R G, Kuhnert W L& Hahn K (2001) Genetics of acid adaptation in oral streptococci. Crit. Rev. Oral Biol. Med. 12: 301–314.
Rallu F, Gruss A& Maguin E (1996) Lactococcus lactis and stress. Antonie van Leeuwenhoek 70: 243–251.
Rallu F, Gruss A, Ehrlich S D& Maguin E (2000) Acid-and multistress-resistant mutants of Lactococcus lactis: identification of intracellular stress signals. Mol. Microbiol. 35: 517–528.
Rao N N& Kornberg A (1999) Inorganic polyphosphate regulates responses of Escherichia coli to nutritional stringencies, environmental stresses and survival in the stationary phase. Prog. Mol. Subcell. Biol. 23: 183–195.
Rastogi V K& Girvin M E (1999) Structural changes linked to proton translocation by subunit c of the ATP synthase. Nature 402: 263–268.
Renault P, Gaillardin C& Heslot H (1988) Role of malolactic fermentation in lactic acid bacteria. Biochimie 70: 375–379.
Rince A, Flahaut S& Auffray Y (2000) Identification of general stress genes in Enterococcus faecalis. Int. J. Food Microbiol. 55: 87–91.
Roméo Y, Gotierrez C&Mistou M Y (2002) Osmoregulation in Lactococcus lactis. BusR, a transcriptional repressor of the glycine betaine uptake system BusA. Submitted for publication.
Russell D W& Setchell K D (1992) Bile acid biosynthesis. Biochemistry 31: 4737–4749.
Sakamoto K, Margolles A, van Veen H W& Konings W N (2001) Hop resistance in the beer spoilage bacterium Lactobacillus brevis is mediated by the ATP-binding cassette multidrug transporter HorA. J. Bacteriol. 183: 5371–5375.
Salema M, Lolkema J S, San Romao M V& Lourero Dias M C (1996) The proton motive force generated in Leuconostoc oenos by L-malate fermentation. J. Bacteriol. 178: 3127–3132.
Salotra P, Singh D K, Seal K P, Krishna N, Jaffe H& Bhatnagar R (1995) Expression of DnaK and GroEL homologs in Leuconostoc esenteroides in response to heat shock, cold shock or chemical stress. FEMS Microbiol. Lett. 131: 57–62.
Sambongi Y, Iko Y, Tanabe M, Omote H, Iwamoto-Kihara A, Ueda I, Yanagida T, Wada Y& Futai M (1999) Mechanical rotation of the c subunit oligomer in ATP synthase (F0F1): direct observation. Science 286: 1722–1724.
Sami M, Yamashita H, Hirono T, Kadokura H, Kitamoto K, Yoda K& Yamasaki M (1997) Hop-resistant Lactobacillus brevis contains a novel plasmid harboring a multidrug resistance-like gene. J. Ferment. Bioeng. 84: 1–6.
Sancar A (1996) DNA excision repair. Annu. Rev. Biochem. 65: 43–81.
Sanders J W, Leenhouts K J, Haandrikman A J, Venema G& Kok J (1995) Stress response in Lactococcus lactis: cloning, expression analysis, and mutation of the lactococcal superoxide dismutase gene. J. Bacteriol. 177: 5254–5260.
Sanders J W, Leenhouts K, Burghoorn J, Brands J R, Venema G& Kok J (1998a) A chloride-inducible acid resistance mechanism in Lactococcus lactis and its regulation. Mol. Microbiol. 27: 299–310.
Sanders J W, Venema G, Kok J& Leenhouts K (1998b) Identification of a sodium chloride-regulated promoter in Lactococcus lactis by single-copy chromosomal fusion with a reporter gene. Mol. Gen. Genet. 257: 681–685.
Sanders J W, Venema G, Kok J (1999) Environmental stress responses in Lactococcus lactis. FEMS Microbiol. Rev. 23: 483–501.
Schiffrin E J& Blum S (2001) Food processing: probiotic microorganisms for beneficial foods. Curr. Opin. Biotechnol. 12: 499–502.
Schmidt G, Hertel C& Hammes W P (1999) Molecular characterisation of the dnaK operon of Lactobacillus sakei LTH681. Syst. Appl. Microbiol. 22: 321–328.
Scott C, Guest J R& Green J (2000a) Characterization of the Lactococcus lactis transcription factor FlpA and demonstration of an in vitro switch. Mol. Microbiol. 35: 1383–1393.
Scott C, Rawsthorne H, Upadhyay M, Shearman C A, Gasson M J, Guest J R& Green J (2000b) Zinc uptake, oxidative stress and the FNR-like proteins of Lactococcus lactis. FEMS Microbiol. Lett. 192: 85–89.
Shibata C, Ehara T, Tomura K, Igarashi K& Kobayashi H (1992) Gene structure of Enterococcus hirae (Streptococcus faecalis) F1F0-ATPase, which functions as a regulator of cytoplasmic pH. J. Bacteriol. 174: 6117–6124.
Sijpesteijn A K (1970) Induction of cytochrome formation and stimulation of oxidative dissimilation by hemin in Streptococcus lactis and Leuconostoc mesenteroides. Antonie Van Leeuwenhoek 36: 335–348.
Skinner M M& Trempy J E (2001) Expression of clpX, an ATPase subunit of the Clp protease, is heat and cold shock inducible in Lactococcus lactis. J. Dairy Sci. 84: 1783–1785.
Small P L& Waterman S R (1998) Acid stress, anaerobiosis and gadCB: lessons from Lactococcus lactis and Escherichia coli. Trends Microbiol. 6: 214–216.
Smeds A, Varmanen P& Palva A (1998) Molecular characterization of a stress-inducible gene from Lactobacillus helveticus. J. Bacteriol. 180: 6148–6153.
Smith A J, Quivey Jr. R G& Faustoferri R C (1996) Cloning and nucleotide sequence analysis of the Streptococcus mutans membrane-bound, proton-translocating ATPase operon. Gene 183: 87–96.
Somero G N (1995) Proteins and temperature. Annu. Rev. Physiol. 57: 43–68.
Spiess C, Beil A& Ehrmann M (1999) A temperature-dependent switch from chaperone to protease in a widely conserved heat shock protein. Cell 97: 339–347.
Steiner K& Malke H (2000) Life in protein-rich environments: the relA-independent response of Streptococcus pyogenes to amino acid starvation. Mol. Microbiol. 38: 1004–1016.
Steiner K& Malke H (2001) relA-Independent amino acid starvation response network of Streptococcus pyogenes. J. Bacteriol. 183: 7354–7364.
Stewart E J, Aslund F& Beckwith J (1998) Disulfide bond formation in the Escherichia coli cytoplasm: an in vivo role reversal for the thioredoxins. EMBO J. 17: 5543–5550.
Stiles M E (1996) Biopreservation by lactic acid bacteria. Antonie Van Leeuwenhoek 70: 331–345.
Stock D, Leslie A G& Walker J E (1999) Molecular architecture of the rotary motor in ATP synthase. Science 286: 1700–1705.
Stortz G& Hengge-Aronis R (Eds.) (2000) Bacterial Stress Responses (p 485). ASM Press, Washington, DC.
Stuart M R, Chou L S& Weimer B C (1999) Influence of carbohydrate starvation and arginine on culturability and amino acid utilization of Lactococcus lactis subsp. lactis. Appl. Environ. Microbiol. 65: 665–673.
Suzuki T, Unemoto T& Kobayashi H (1988) Novel streptococcal mutants defective in the regulation of H+-ATPase biosynhesis and in F0 complex. J. Biochem. Chem. 263: 11840–11843
Svensater G, Sjogreen B& Hamilton I R (2000) Multiple stress responses in Streptococcus mutans and the induction of general and stress-specific proteins. Microbiology 146: 107–117.
Takahashi N& Yamada T (1999) Acid-induced acid tolerance and acidogenicity of non-mutans streptococci. Oral Microbiol. Immunol. 14: 43–48.
Teixera P, Castro H, Mohacsi-Farkas C& Kirby R (1997) Identification of sites of injury in Lactobacillus bulgaricus during heat stress. J. Appl. Microbiol. 83: 219–226.
ten Brink B, Otto R, Hansen U P& Konings W N (1985) Energy recycling by lactate efflux in growing and nongrowing cells of Streptococcus cremoris. J. Bacteriol. 162: 383–390.
Tettelin H, Nelson K E, Paulsen I T, Eisen J A, Read T D, Peterson S, Heidelberg J, DeBoy R T, Haft D H, Dodson R J, Durkin A S, Gwinn M, Kolonay J F, Nelson W C, Peterson J D, Umayam L A, White O, Salzberg S L, Lewis M R, Radune D, Holtzapple E, Khouri H, Wolf A M, Utterback T R, Hansen C L, McDonald L A, Feldblyum T V, Angiuoli S, Dickinson T, Hickey E K, Holt I E, Loftus B J, Yang F, Smith H O, Venter J C, Dougherty B A, Morrison D A, Hollingshead S K& Fraser C M (2001) Complete genome sequence of a virulent isolate of Streptococcus pneumoniae. Science 293: 498–506.
Thammavongs B, Corroler D, Panoff J M, Auffray Y& Boutibonnes P (1996) Physiological response of Enterococcus faecalis JH2-2 to cold shock: growth at low temperatures and freezing/thawing challenge. Lett. Appl. Microbiol. 23: 398–402.
Thanassi D G, Cheng L W& Nikaido H (1997) Active efflux of bile salts by Escherichia coli. J. Bacteriol. 179: 2512–2518.
Tonon T& Lonvaud-Funel A (2000) Metabolism of arginine and its positive effect on growth and revival of Oenococcus oeni. J. Appl. Microbiol. 89: 526–531.
Tourdot-Marechal R, Fortier L C, Guzzo J, Lee B& Divies C (1999) Acid sensitivity of neomycin-resistant mutants of Oenococcus oeni: a relationship between reduction of ATPase activity and lack of malolactic activity. FEMS Microbiol. Lett. 178: 319–326.
Trainor V C, Udy R K, Bremer P J& Cook G M (1999) Survival of Streptococcus pyogenes under stress and starvation. FEMS Microbiol. Lett. 176: 421–428.
Turner M S, Woodberry T, Hafner L M& Giffard P M (1999) The bspA locus of Lactobacillus fermentum BR11 encodes an L-cystine uptake system. J. Bacteriol. 181: 2192–2198.
Uguen P, Hamelin J, Le Pennec J P& Blanco C (1999) Influence of osmolarity and the presence of an osmoprotectant on Lactococcus lactis growth and bacteriocin production. Appl. Environ. Microbiol. 65: 291–293.
van Asseldonk M, Simons A, Visser H, de Vos W M& Simons G (1993) Cloning, nucleotide sequence, and regulatory analysis of the Lactococcus lactis dnaJ gene. J. Bacteriol. 175: 1637–1644.
Van de Guchte M, Ehrlich S D& Maguin E (2001) Production of growth-inhibiting factors by Lactobacillus delbrueckii. J. Appl. Microbiol. 91: 147–153.
Van der Heide T& Poolman B (2000a) Glycine betaine transport in Lactococcus lactis is osmotically regulated at the level of expression and translocation activity. J. Bacteriol. 182: 203–206.
Van der Heide T& Poolman B (2000b) Osmoregulated ABCtransport system of Lactococcus lactis senses water stress via changes in the physical state of the membrane. Proc. Natl. Acad. Sci. U.S.A. 97: 7102–7106.
Van der Heide T, Stuart M C& Poolman B (2001) On the osmotic signal and osmosensing mechanism of an ABC transport system for glycine betaine. EMBO J. 20: 7022–7032.
van Veen HW, Putman M, Margolles A, Sakamoto K& Konings W N (1999) Structure-function analysis of multidrug transporters in Lactococcus lactis. Biochim. Biophys. Acta 1461: 201–206.
van Velkinburgh J C& Gunn J S (1999) PhoP-PhoQ-regulated loci are required for enhanced bile resistance in Salmonella spp. Infect. Immun. 67: 1614–1622.
VanBogelen R A, Greis K D, Blumenthal M, Tani T H& Matthews R (1999) Mapping regulatory networks in microbial cells. Trends Microbiol. 7: 320–328.
VanBogelen R A& Neidhardt F (1990) Ribosomes as sensors of heat and cold shock in Escherichia coli. Proc. Natl. Acad. Sci. U.S.A. 87: 5589–5593.
Varmanen P, Ingmer H& Vogensen F K (2000) ctsR of Lactococcus lactis encodes a negative regulator of clp gene expression. Microbiology 146: 1447–1455.
Voelker U, Voelker A, Maul B, Hecker M, Dufour A& Haldenwang W G (1995) Separate mechanisms activate sigma B of Bacillus subtilis in response to environmental and metabolic stresses. J. Bacteriol. 177: 3771–3780.
Volker U, Andersen K K, Antelmann H, Devine K M& Hecker M (1998) One of two osmC homologs in Bacillus subtilis is part of the sigmaB-dependent general stress regulon. J. Bacteriol. 180: 4212–4218.
Walker D C, Girgis H S& Klaenhammer T R (1999) The groESL chaperone operon of Lactobacillus johnsonii. Appl. Environ. Microbiol. 65: 3033–3041.
Wang N, Yamanaka K& Inouye M (1999) CspI, the ninth member of the CspA family of Escherichia coli, is induced upon cold shock. J. Bacteriol. 181: 1603–1609.
Weber M H, Beckering C L& Marahiel M A (2001a) Complementation of cold shock proteins by translation initiation factor IF1 in vivo. J. Bacteriol. 183: 7381–7386.
Weber M H, Volkov A V, Fricke I, Marahiel M A& Graumann P L (2001b) Localization of cold shock proteins to cytosolic spaces surrounding nucleoids in Bacillus subtilis depends on active transcription. J. Bacteriol. 183: 6435–6443.
Wehmeier L, Schafer A, Burkovski A, Kramer R, Mechold U, Malke H, Puhler A& Kalinowski J (1998) The role of the Corynebacterium glutamicum rel gene in (p)ppGpp metabolism. Microbiology 144: 1853–1862.
Wells J M, Robinson K, Chamberlain L M, Schofield K M& Le Page R W (1996) Lactic acid bacteria as vaccine delivery vehicles. Antonie Van Leeuwenhoek 70: 317–330.
Wendrich T M& Marahiel M A (1997) Cloning and characterization of a relA/spoT homologue from Bacillus subtilis. Mol. Microbiol. 26: 65–79.
Whitaker R D& Batt C A (1991) Characterization of the heat shock response in Lactococcus lactis subsp. lactis. Appl. Environ. Microbiol. 57: 1408–1412.
Whitehead K E, Webber G M& England R R (1998) Accumulation of ppGpp in Streptococcus pyogenes and Streptococcus rattus following amino acid starvation. FEMS Microbiol. Lett. 159: 21–26.
Wilkins J C, Homer K A& Beighton D (2001) Altered protein expression of Streptococcus oralis cultured at low pH revealed by two-dimensional gel electrophoresis. Appl. Environ. Microbiol. 67: 3396–3405.
Willimsky G, Bang H, Fischer G& Marahiel M A (1992) Characterization of cspB, a Bacillus subtilis inducible cold shock gene affecting cell viability at low temperatures. J. Bacteriol. 174: 6326–6335.
Wouters J A, Frenkiel H, de Vos W M, Kuipers O P& Abee T (2001) Cold shock proteins of Lactococcus lactis MG1363 are involved in cryoprotection and in the production of cold-induced proteins. Appl. Environ. Microbiol. 67: 5171–5178.
Wouters J A, Sanders J W, Kok J, de Vos W M, Kuipers O P& Abee T (1998) Clustered organization and transcriptional analysis of a family of five csp genes of Lactococcus lactis MG1363. Microbiology 144: 2885–2893.
Wouters J A, Jeynov B, Rombouts F M, de Vos W M, Kuipers O P& Abee T (1999a) Analysis of the role of 7 kDa cold-shock proteins of Lactococcus lactis MG1363 in cryoprotection. Microbiology 145: 3185–3194.
Wouters J A, Rombouts F M, de Vos W M, Kuipers O P& Abee T (1999b) Cold shock proteins and low-temperature response of Streptococcus thermophilus CNRZ302. Appl. Environ. Microbiol. 65: 4436–4442.
Wouters J A, Kamphuis H H, Hugenholtz J, Kuipers O P, de Vos W M& Abee T (2000a) Changes in glycolytic activity of Lactococcus lactis induced by low temperature. Appl. Environ. Microbiol. 66: 3686–3691.
Wouters J A, Mailhes M, Rombouts F M, de Vos W M, Kuipers O P& Abee T (2000b) Physiological and regulatory effects of controlled overproduction of five cold shock proteins of Lactococcus lactis MG1363. Appl. Environ. Microbiol. 66: 3756–3763.
Wouters J A, Rombouts F M, Kuipers O P, de Vos W M& Abee T (2000c) The role of cold-shock proteins in low-temperature adaptation of food-related bacteria [In Process Citation]. Syst. Appl. Microbiol. 23: 165–173.
Xia B, Ke H& Inouye M (2001) Acquirement of cold sensitivity by quadruple deletion of the cspA family and its suppression by PNPase S1 domain in Escherichia coli. Mol.Microbiol. 40: 179–188.
Yamamoto N, Masujima Y& Takano T (1996) Reduction of membrane bound ATPase activity in a Lactobacillus helveticus strain with slower growth at low pH. FEMS Microbiol. Rev. 138: 179–184.
Yamanaka K, Fang L& Inouye M (1998) The CspA family in Escherichia coli: multiple gene duplication for stress adaptation. Mol. Microbiol. 27: 247–255.
Yamanaka K, Mitta M& Inouye M (1999) Mutation analysis of the 5' untranslated region of the cold shock cspA mRNA of Escherichia coli. J. Bacteriol. 181: 6284–6291.
Yamashita Y, Takehara T& Kuramitsu H K (1993) Molecular characterization of a Streptococcus mutans mutant altered in environmental stress responses. J. Bacteriol. 175: 6220–6228.
Yi X, Kot E& Bezkorovainy A (1998) Properties of NADH oxidase from Lactobacillus delbrueckii ssp. bulgaricus. J. Sci. Food Agric. 78: 527–534.
Yokota A, Amachi S, Ishii S& Tomita F (1995) Acid sensitivity of a mutan of Lactococcus lactis subsp. lactis C2 with reduced membrane bound ATPase activity. Biosci. Biotech. Biochem. 59: 2004–2007.
Yokota A, Veenstra M, Kurdi P, van Veen H W& Konings W N (2000) Cholate resistance in Lactococcus lactis is mediated by an ATP-dependent multispecific organic anion transporter. J. Bacteriol. 182: 5196–5201.
Yura T, Kanemori M& Morita T M (2000) The heat shock response: regulation and function. In: Storz G& Hengge-Aronis R (Eeds.) Bacterial Stress Responses (pp 3–18). ASM Press, Washington, DC.
Zhu M, Takenaka S, Sato M& Hoshino E (2001) Influence of starvation and biofilm formation on acid resistance of Streptococcus mutans. Oral Microbiol. Immunol. 16: 24–27.
Zuber U& Schumann W (1994) CIRCE, a novel heat shock element involved in regulation of heat shock operon dnaK of Bacillus subtilis. J. Bacteriol. 176: 1359–1363.
Zuniga M, Champomier-Verges M, Zagorec M& Perez-Martinez G (1998) Structural and functional analysis of the gene cluster encoding the enzymes of the arginine deiminase pathway of Lactobacillus sake. J. Bacteriol. 180: 4154–4159.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
van de Guchte, M., Serror, P., Chervaux, C. et al. Stress responses in lactic acid bacteria. Antonie Van Leeuwenhoek 82, 187–216 (2002). https://doi.org/10.1023/A:1020631532202
Issue Date:
DOI: https://doi.org/10.1023/A:1020631532202