Abstract
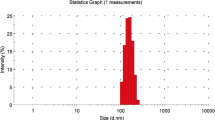
An animal study was carried out to evaluate the in vivo bronchodilator action of isoproterenol (Iso) from poly(glycolide-co-lactide) (PGL) microspheres. Microspheres with a mean diameter of 4.5 µm and a drug load of 7% were administered intratracheally to Long-Evans rats. The microspheres released about 70% of the incorporated drug in the instillation medium before administration, which provided immediate action, and the remaining 30% was available for sustained release. A total of 120 animals was anesthetized, paralyzed, artificially ventilated, and divided into 15 groups (n = 8): 3 groups each for saline, blank microspheres, free Iso, blank microspheres with free Iso, and microencapsulated Iso. All instillations were made in a volume of 1 ml/kg and the dose of all Iso preparations was 0.1 mg/kg. At 3, 6, or 12 hr after the intratracheal instillation, a serotonin challenge (40 µg/rat) was administered intravenously to constrict the airways. Airway function tests were performed at each time interval on one group of animals by a maximal expiratory flow-volume maneuver. The heart rate in animals receiving Iso formulations was similar to that in the saline control group, indicating minimal systemic effect of the dose administered. The systemic serum levels were below 2 ng/ml in all the groups. Animals receiving encapsulated Iso resisted the serotonin challenge for at least 12 hr after intratracheal instillation, indicating that the drug was still present over this period of time. On the other hand, the serotonin-induced airway constriction observed in the animals receiving blank microspheres, free Iso, or free Iso with blank microspheres was similar to that in saline controls at all time points. The results clearly show that only a small fraction of the free dose is required in sustained-release form for a prolonged pharmacological effect, resulting in a 50- to 100-fold reduction in the total dose administered.
Similar content being viewed by others
REFERENCES
I. Gonda. Aerosols for delivery of therapeutic and diagnostic agents to the respiratory tract. Crit. Rev. Drug Deliv. Syst. 6:273–313 (1990).
T. J. H. Clark. Effect of beclomethasone dipropionate delivered by aerosol in patients with asthma. Lancet 6:1361–1364 (1972).
A. Neville, J. B. D. Palmer, J. Gaddie, C. S. May, K. N. V. Palmer, and L. E. Murchison. Metabolic effects of salbutamol: Comparison of aerosol and intravenous administration. Br. Med. J. 1:413–414 (1977).
R. G. Taylor. Adrenoceptor agonist and steroids in respiratory disease. In D. Ganderton and T. Jones (eds.), Drug Delivery to Respiratory Tract, Ellis Horwood, Chichester, 1987, pp. 27–36.
S. D. Anderson, J. P. Seale, P. Rozea, L. Bandler, G. Theobald, and D. A. Lindsay. Inhaled and oral salbutamol in exercise induced asthma. Am. Rev. Resp. Dis. 114:493–500 (1976).
Y. F. J. Choo-Kang, W. T. Simpson, and I. W. B. Grant. Controlled comparison of the bronchodilator effects of three b-adrenergic stimulant drugs administered by inhalation to patients with asthma. Br. Med. J. 2:287–289 (1969).
USP DI Vol. I, Drug Information for Health Care Provider, 5th ed., 1985, pp. 58–59, 716–719.
D. J. Riley, R. T. Liu, and N. H. Edelman. Enhanced response to aerosolized bronchodilator therapy in asthma using respiratory maneuvers. Chest 76:501–507 (1979).
D. Heimer, C. Shim, and M. H. Williams, Jr. The effect of sequential inhalations of metaproterenol aerosol in asthma. J. Allergy Clin. Immunol. 66:75–77 (1980).
T. A. McCalden, R. M. Abra, and P. J. Mihalko. Bronchodilator efficiency of liposome formulations of metaproterenol sulfate in the anesthetized guinea pig. J. Liposome Technol. 1:211–222 (1989).
Y. L. Lai. Maximum expiratory flow in guinea pig. Lung 166:303–313 (1988).
P. P. DeLuca, A. J. Hickey, M. Kanke, A. M. Hazrati, P. Wedlund, and F. Rypacek. Topics in Pharmaceutical Sciences, Elsevier, Amsterdam, 1987.
P. P. DeLuca. U.S. Patent 4,818,542, April 4 (1988).
T. Sato, H. G. Schroeder, M. Kanke, and P. P. DeLuca. Porous biodegradable microspheres for controlled drug delivery. I. Assessment of processing conditions and solvent removal techniques. Pharm. Res. 5:21 (1988).
F. A. J. Van Der Hoorn et al. Determination of catecholamines in human plasma by high performance liquid chromatography: Comparison between a new method with fluorescence detection and an established method with electrochemical detection. J. Chromatogr. 487:17–28 (1989).
A. Mitushi et al. High performance liquid chromatography of plasma catecholamines using 1,2-diphenylethylenediamine as a precolumn fluorescence derivatization reagent. J. Chromatogr. 344:61–70 (1985).
A. Nohta et al. High sensitivity determination of isoproterenol in plasma and urine by high performance liquid chromatography with fluorescence detection. Bunseki Kagaku 35:288–292 (1990).
N. Svedmyr and G. Thiringer. The effects of salbutamol and isoprenaline on beta-receptors in patients with chronic obstructive lung disease. Postgrad. Med. J. 47:44–46 (1971).
J. D. Brian, P. A. Valberg, and S. Sneddon. Mechanisms of aerosol deposition and clearance. In F. Moren, M. T. Newhouse, and M. B. Dolovich (eds.), Aerosols in Medicine, Principles, Diagnosis and Therapy, Elsevier, Amsterdam, 1985, pp. 123–141.
P. J. Barnes and N. B. Pride. Dose-response curves to inhaled β-adrenoceptor agonist in normal and asthmatic subjects. Br. J. Clin. Pharmacol. 15:677–682 (1983).
M. H. Williams, Jr. and C. Kane. Dose response of patients with asthma to isoproterenol. Am. Rev. Resp. Dis. 111:321–324 (1975).
R. Gryglewski and J. R. Vane. The inactivation of noradrenalin and isoprenalin in dogs. Br. J. Pharmacol. 39:573–584 (1970).
Associated Press. New York Times, Thursday, August 8 (1991).
M. K. Kulkarni, V. A. Philip, W. A. Doll, R. C. Mehta, G. A. Digenis, and P. P. DeLuca. Aerosol formulations of biodegradable microspheres. Pharm. Res. 8:S86 (1991).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lai, YL., Mehta, R.C., Thacker, A.A. et al. Sustained Bronchodilation with Isoproterenol Poly(Glycolide-co-Lactide) Microspheres. Pharm Res 10, 119–125 (1993). https://doi.org/10.1023/A:1018989400517
Issue Date:
DOI: https://doi.org/10.1023/A:1018989400517