Abstract
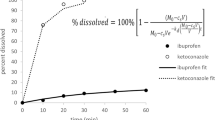
A quantitative analysis of the dependence of dissolution rate on the relative surface area occupied by two non-interacting drug mixtures from co-compressed slabs is described. The results from the experimental dissolution rates of each component from naproxen/ phenytoin co-compressed slabs under laminar flow conditions, when corrected for the area occupied by that component in the slab, contradict the stagnant layer model predictions, where dissolution rates are assumed to be directly proportional to the occupied surface area. Simulations from non-mixed co-compressates of naproxen and phenytoin indicated that dissolution rates are proportional to bL2/3, as reported for pure compounds in the laminar dissolution apparatus by Shah and Nelson. However, for a well mixed co-compressate, which differs with the non-mixed case only in the distribution of particles, this proportionality did not hold. The deviation was explained by ‘carryover’ of material from one section of the component to the next due to fluid flow, resulting in an increase in apparent effective length of the component in the slab (Leff).
Similar content being viewed by others
REFERENCES
A. W. Hixson and S. J. Baum. Mass transfer and chemical reaction in liquid-solid agitation. J. Ind. Eng. Chem. 35:p. 528. (1944)
C. V. King and S. S. Brodie. The rate of dissolution of benzoic acid in dilute aqueous alkali. J. Am. Chem. Soc. 59:p. 1375–1379. (1937)
K. G. Mooney, M. A. Mintun, K. J. Himmelstein and V. J. Stella. Dissolution kinetics of carboxylic acids I: Effect of pH under unbuffered conditions. J. Pharm. Sci. 70:p. 13–22. (1980)
W. I. Higuchi, N. A. Mir, and S. J. Desai. Dissolution rates of polyphase mixtures. J. Pharm. Sci. 54(10):p. 1405–1410. (1965)
G. R. Carmichael, S. A. Shah, and E. L. Parrott. General model for dissolution rates of n-component, non-disintegrating spheres. J. Pharm. Sci. 70(12):p. 1331–1338. (1981)
S. A. Shah and E. L. Parrott. Dissolution of two-component solids. J. Pharm. Sci. 65(12):p. 1784–1790. (1976)
H. Grijseels, D. J. A. Crommelin, and C. J. Blaey. Hydrodynamic approach to dissolution rate. Pharm. Weekblad. Sci. Ed. 3:p. 129–144. (1981)
A. C. Shah and K. G. Nelson. Evaluation of a convective diffusion drug dissolution rate model. J. Pharm. Sci. 64(9):p. 1518–1520. (1975)
S. Neervannan, L. S. Dias, M. Z. Southard and V. J. Stella. A convective diffusion model for dissolution of two noninteracting drug mixtures from co-compressed slabs under laminar hydrodynamic conditions. Pharm. Res. 11:1288–1295. (1994)
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Neervannan, S., Southard, M.Z. & Stella, V.J. Dependence of Dissolution Rate on Surface Area: Is a Simple Linear Relationship Valid for Co-Compressed Drug Mixtures?. Pharm Res 11, 1391–1395 (1994). https://doi.org/10.1023/A:1018979419714
Issue Date:
DOI: https://doi.org/10.1023/A:1018979419714