Abstract
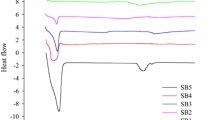
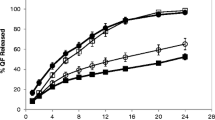
Tablets of acetaminophen as a model drug were prepared with low-substituted hydroxypropylcellulose (L-HPC) of various particle sizes at various loadings in the formulation. Drug release into an aqueous dissolution medium (pH 1.2) was remarkably sustained from tablets prepared with fine L-HPC (LH41) at loadings of more than 20%. Tablets prepared with less than 20% LH41 or with coarse L-HPCs (LH11, LH21, and LH31) disintegrated in the medium, resulting in rapid release of the drug. The difference in behavior could not be explained in terms of differences in tablet strength, but in swelling and water uptake abilities of the tablet's polymer. Swelling work (swelling force), water penetration speed, and water uptake of LH41 (4.4-µm average particle size) were much smaller than those of coarse L-HPCs. The formation of a continuous gel-like layer on the surface of tablets containing more than 20% LH41 was another factor to sustain the drug release rate.
Similar content being viewed by others
REFERENCES
H. Nakagami and M. Nada. Application of micronized insoluble cellulose to sustained release tablets. Proc. Int. Symp. Control. Bioact. Mater. 15:11–12 (1988).
Y. Kawashima, H. Takeuchi, T. Hino, T. Niwa, T. L. Lin, F. Sekigawa, and K. Kawahara. Application of cellulose derivatives to pharmaceutical preparation—Compressibility and drug release characteristics. ROC-Jap. Symp. Pharm. Tech., Taipei, 1989, p. 57.
H. Nogami, T. Nagai, E. Fukuoka, and T. Sonobe. Disintegration of the aspirin tablets containing potato starch and microcrystalline cellulose in various concentrations. Chem. Pharm. Bull. 17:1450–1455 (1969).
E. M. Rudnic, C. T. Rhodes, S. Welch, and P. Bernardo. Evaluations of the mechanism of disintegrant action. Drug. Dev. Ind. Pharm. 8(1):87–109 (1982).
C. Caramella, P. Colombo, U. Conte, F. Ferrari, A. La Manna, H. V. Can Kamp, and G. K. Bolhuis. Water uptake and disintegrating force measurements: Towards a general understanding of disintegration mechanisms. Drug Dev. Ind. Pharm. 12:1749–1766 (1986).
H. V. van Kamp, G. K. Bolhuis, A. H. de Boer, C. F. Lerk, and Lie-A-Huen. The role of water uptake on tablet disintegration. Acta Helv. 61:22–29 (1986).
P. L. Catellani, P. Predella, A. Bellotti, and P. Colombo. Tablet water uptake and disintegration force measurements. Int. J. Pharm. 51:63–66 (1989).
L. S. C. Wan and K. P. P. Prasad. Uptake of water into tablets with low-substituted carboxymethyl cellulose sodium as disintegrant. Int. J. Pharm. 55:115–121 (1989).
P. Colombo, C. Caramella, U. Conte, A. La Manna, A. M. Guyot-Hermann, and J. Ringard. Disintegrating force and tablet properties. Drug Dev. Ind. Pharm. 7:135–153 (1981).
N. A. Peppas and P. Colombo. Development of disintegration forces during water penetration in porous pharmaceutical systems. J. Control. Release 10:245–250 (1989).
E. M. Rudnic, J. M. Lausier, R. N. Chilamkurti, and C. T. Rhodes. Studies of the utility of cross linked polyvinylpolypyrrolidine as a tablet disintegrant. Drug Dev. Ind. Pharm. 6:291–309 (1980).
D. Gissinger and A. Stamn. A comparative evaluation of the properties of some tablet disintegrants. Drug Dev. Ind. Pharm. 6:511–536 (1980).
J. M. Newton, G. Rowley, J. T. Fell, D. G. Peacock, and K. Ridgway. Computer analysis of the relation between tablet strength and compaction pressure. J. Pharm. Pharmacol. 23:195S–201S (1971).
H. Lapidus and N. G. Lordi. Drug release from compressed hydrophilic matrices. J. Pharm. Sci. 57:1292–1301 (1968).
D. A. Alderman. A review of cellulose ethers in hydrophilic matrices for oral controlled-release dosage forms. Int. J. Pharm. Tech. Prod. Mfr. 5(3):1–9 (1984).
F. Ferrari, S. Rossi, M. Bertoni, C. Caramella, and M. Geddo. Modelling of water penetration into fast disintegrating tablets. STP Pharma Sci. 1(2):137–144 (1991).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kawashima, Y., Takeuchi, H., Hino, T. et al. Low-Substituted Hydroxypropylcellulose as a Sustained-Drug Release Matrix Base or Disintegrant Depending on Its Particle Size and Loading in Formulation. Pharm Res 10, 351–355 (1993). https://doi.org/10.1023/A:1018975919598
Issue Date:
DOI: https://doi.org/10.1023/A:1018975919598