Abstract
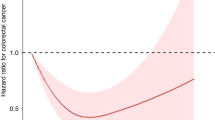
Experimental and human epidemiologic data suggest a protective rolefor vitamin D in large bowel cancer. To investigate this association, weconducted a nested case-control study within a Finnish clinical trial cohort.Cases (n = 146) were participants diagnosed with primary adenocarcinoma ofthe large bowel. Controls were matched (2:1) to cases on age, date ofbaseline blood draw, and study clinic. Prediagnostic serum levels of thevitamin D metabolites, 25-hydroxyvitamin D (25-OH D), and1,25-dihydroxyvitamin D (1,25-DIOHD) were used as primary exposure measures.The baseline geometric-mean serum level of 25-OH D was 11.6 percent lower incases than in controls (12.2 cf 13.8 ug/l, P = 0.01) while serum levels of1,25-DIOH D did not differ by case-control status. No association was seenbetween serum levels of 1,25-DIOH D and large bowel cancer risk. However, theestimated relative risk (RR) of large bowel cancer decreased with increasinglevel of serum 25-OH D and the associa tion was more pronounced for rectalcancer (55 cases; RR by quartile = 1.00, 0.93, 0.77, 0.37; trend P = 0.06).Neither exclusion of early cases nor multivariate adjustment for potentialconfounders materially altered these estimates. There was no evidence ofeffect modification by level of 1,25-dihydroxyvitamin D or with other knownrisk-factors for large bowel cancer.
Similar content being viewed by others
References
American Cancer Society. Cancer Facts and Figures-1994. Atlanta, GA (USA): ACS, 1994.
Miller BA, Gloeckler-Ries LA, Hankey BF, et al. SEER Cancer Statistics Review: 1973-1990. Bethesda, MD (USA): National Cancer Institute, 1993.
Hakulinen T, Kenward M, Luostarinen T, et al. Cancer in Finland in 1954-2008: incidence, mortality and prevalence by region. Helsinki: Finnish Foundation for Cancer Research, 1989.
Garland CF, Garland FC. Do sunlight and vitamin D reduce the likelihood of colon cancer? Int J Epidemiol {dy1980}; 9: 227–31.
Gorham ED, Garland CF, Garland FC. Acid haze air pollution and breast and colon cancer mortality in 20 Canadian cities. Can J Public Health {dy1989}; 80: 96–100.
Emerson JC, Weiss NS. Colorectal cancer and solar radiation. Cancer Causes Control {dy1992}; 3: 95–9.
Garland CF, Shekelle RB, Barrett-Connor E, Criqui MH, Rossof AH, Paul O. Dietary vitamin D and calcium and risk of colorectal cancer: a 19 year prospective study in men. Lancet {dy1985}; 1: 307–9.
Bostick RM, Potter JD, Sellers TA, McKenzie DR, Kushi LH, Folsom AR. Relation of calcium, vitamin D, and dairy food intake to incidence of colon cancer among older women. Am J Epidemiol {dy1993}; 137: 1302–17.
Garland CF, Garland FC, Ko Shaw E, Gorham ED, Comstock GW, Helsing KJ. Serum 25-hydroxyvitamin D and colon cancer: eight year prospective study. Lancet {dy1989}; 2: 1176–8.
Braun MM, Helzlsouer KJ, Hollis BW, Comstock GW. Colon cancer and prediagnostic serum 25-D and 1,25-D levels [Abstract]. In: Norman AW, Bouillon R, Thomasset M, eds. Vitamin D. A Pluripotent Steroid Hormone: Structural Studies, Molecular Endocrinology, and Clinical Applications. Berlin, Germany: de Gruyter, 1994: 494–5.
Garland CF, Garland FC, Gorham ED. Can colon cancer incidence and death rates be reduced with calcium and vitamin D? Am J Clin Nutr {dy1991}; 54: 193s–201s.
Garland CF, Garland FC, Gorham ED. Colon cancer parallels rickets. In: Lipkin M, Newmark HL, Kelloff G, eds. Calcium, Vitamin D and Prevention of Colon Cancer. Boca Raton, Fl (USA): CRC Press, 1991: 81–108.
Ross TK, Darwish HM, DeLuca HF. Molecular biology of vitamin D action. Vitam Horm {dy1994}; 49: 281–326.
Haussler MR, McCain TA. Basic and clinical concepts related to vitamin D metabolism and action. NEJM {dy1977}; 297: 974–83.
Reichel H, Koeffler HP, Norman AW. The role of the vitamin D endocrine system in health and disease. NEJM {dy1989}; 320: 980–91.
DeLuca HF. Vitamin D Metabolism and Function. New York,NY (USA): Springer-Verlag, 1979.
Fraser DR. Vitamin D. Lancet {dy1995}; 345: 104–7.
Darwish H, DeLuca HF. Vitamin D-regulated gene expression. Crit Rev Eukar Gene Express {dy1993}; 3: 89–116.
Abe E, Miyaura H, Sakagami M, et al. Differentiation of mouse myeloid leukemia cells induced by 1, alpha-25-dihydroxyvitamin D3. Proc Natl Acad Sci {dy1981}; 78: 4990–4.
Colston KW, Colston MJ, Feldman D. 1,25-dihydroxyvitamin D3 and malignant melanoma: the presence of receptors and inhibition of cell growth in culture. Endocrinology {dy1981}; 108: 1083–6.
Frampton RJ, Suva LJ, Eisman JA, et al. Presence of 1,25-dihydroxyvitamin D3 receptors in established human cancer cell lines in culture. Cancer Res {dy1982}; 42: 1116–9.
Lointier P, Wargovich MJ, Saez S, Levin B, Wildrick DM, Boman BM. The role of vitamin D3 in the proliferation of a human colon cancer cell line in vitro. Anticancer Res {dy1987}; 7: 817–22.
Brehier A, Thomasset M. Human colon cell line HT-29: characterization of the 1,25-dihydroxyvitamin D3 receptor and induction of differentiation by the hormone. J Steroid Biochem {dy1988}; 29: 265–70.
Harper KD, Iozzo RV, Haddad JG. Receptors for and bioresponses to 1,25-dihydroxyvitaminDin a human colon carcinoma cell line. Metabolism {dy1989}; 38: 1062–9.
Cross HS, Huber C, Peterlik M. Antiproliferative effect of 1,25-dihydroxyvitamin D3 and its analogs on human colon adenocarinoma cells (CACO-2): influence of extracellular calcium. Biochem Biophys Res Commun {dy1991}; 179: 57–62.
Thomas MG, Tebbutt S, Williamson RCN. Vitamin D and its metabolites inhibit cell proliferation in human rectal mucosa and a colon cancer cell line. Gut {dy1992}; 33: 1660–3.
Shabahang M, Buras RR, Davoodi F, Schumaker LM, Nauta RJ, Evans SR. 1,25-dihydroxyvitamin D3 receptor as a marker of human colon carcinoma cell line differentiation and growth inhibition. Cancer Res {dy1993}; 53: 3712–8.
Pence BC, Buddingh F. Inhibition of dietary fat-promoted colon carcinogenesis in rats by supplemental calcium or vitamin D3. Carcinogenesis {dy1988}; 9: 187–90.
Sitrin MD, Halline AG, Abrahams C, Brasitus TA. Dietary calcium and vitamin D modulate 1,2-dimethylhydrazineinduced colonic carcinogenesis in the rat [Abstract]. Cancer Res {dy1991}; 51: 5608–13.
Beaty MM, Lee EY, Glauert HP. Influence of dietary calcium and vitamin D on colon epithelial cell proliferation and 1,2-dimethylhydrazine-induced colon carcinogenesis in rats fed high fat diets [Abstract]. J Nutr {dy1993}; 123: 144–52.
Batchelor AJ, Compston JE. Reduced plasma half-life of radio-labelled 25-hydroxyvitamin D3 in subjects receiving a high-fibre diet. Br J Nutr {dy1983}; 49: 213–6.
Punnonen R, Gillespy M, Hahl M, Koskinen T, Notelovitz M. Serum 25-OHD, Vitamin A and vitamin E concentrations in healthy Finnish and Floridian women. Int J Vit Nutr Res {dy1987}; 58: 37–9.
Holick MF. Environmental factors that influence the cutaneous production of vitamin D. Am J Clin Nutr {dy1995}; 61(Suppl.): 638s–45s.
Lamberg-Allardt C. Vitamin D intake, sunlight exposure and 25-hydroxyvitamin D levels in the elderly during one year. Ann Nutr Metab {dy1984}; 28: 144–50.
Beadle PC, Burton JL, Leach JF. Correlation of seasonal variation of 25-hydroxycholecalciferol with UV radiation dose. Br J Dermatol {dy1980}; 103: 289–93.
Holmberg I, Larsson A. Seasonal variation of vitamin D3 and 25-hydroxyvitamin D3 in human serum. Clin Chim Acta {dy1980}; 100: 173–4.
Sowers JR, Wallace RB, Hollis BW, Lemke JH. Parameters related to 25-OH-D levels in a population-based study of women. Am J Clin Nutr {dy1986}; 43: 621–8.
Holick MF. The use and interpretation of assays for vitamin D and its metabolites. J Nutr {dy1990}; 120: 1464–9.
The Alpha-Tocopherol Beta Carotene Cancer Prevention Study Group. The alpha-tocopherol, beta carotene lung cancer prevention trial: design, methods, participant characteristics, and compliance. Ann Epidemiol {dy1994}; 4: 1–9.
The Alpha-Tocopherol Beta Carotene Cancer Prevention Study Group. The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. NEJM {dy1994}; 330: 1029–35.
World Health Organization. International Classification of Diseases, Ninth Revision. Geneva, Switzerland: WHO, 1997.
Kyllonen LEJ, Teppo L, Lehtonen M. Completeness and accuracy of registration of colorectal cancer in Finland. Ann Chir Gynaecol {dy1987}; 76: 185–90.
Pearce N. Incidence density matching with a simple SAS computer program. Int J Epidemiol {dy1989}; 18: 981–4.
Hollis BW, Kamerud JQ, Selvaag SR, Lorenz JD, Napoli JL. Determination of vitamin D status by radioimmuno assay with an125I-labeled tracer. Clin Chem {dy1993}; 39: 529–33.
Hollis BW. Assay of circulating 1,25-dihydroxyvitamin D involving a novel single-cartridge extraction and purification procedure. Clin Chem {dy1986}; 32: 2060–3.
Pietinen P, Hartman AM, Haapa E, et al. Reproducibility and validity of dietary assessment instruments: 1. A selfadministered food use questionnaire with a portion size booklet. Am J Epidemiol {dy1988}; 128: 655–66.
Devesa SS, Chow WH. Variation in colorectal cancer incidence in the United States by subsite of origin. Cancer {dy1993}; 71: 3819–26.
Delattre O, Olschwang S, Law DJ, et al. Multiple genetic alterations in distal and proximal colorectal cancer. Lancet {dy1989}; 2: 353–6.
Stewart RJ, Stewart AW, Turnbull PRG, Isbister WH. Sex differences in subsite incidence of large bowel cancer. Dis Colon Rectum {dy1983}; 26: 658–60.
Bufill J. Colorectal cancer: evidence for distinct genetic categories based on proximal or distal tumor location. Ann Intern Med {dy1990}; 113: 779–88.
Offerhaus GJA, Giardiello FM, Tersmette KWF, et al. Ethnic differences in the anatomical location of colorectal adenomatous polyps. Int J Cancer {dy1991}; 49: 641–4.
Ponz de Leon M, Sachhetti C, Sassatelli R, Zanghieri G, Roncucci L, Scalmati A. Evidence for the existence of different types of large bowel tumor: suggestions from the clinical data of a population-based registry. J Surg Oncol {dy1990}; 44: 35–43.
Faivre J, Bedenne L, Boutron MC, Milan C, Collonges R, Arveux P. Epidemiological evidence for distinguishing subsites of colorectal cancer. J Epidemiol Community Health {dy1989}; 43: 356–61.
Launoy G, Pottier D, Gignoux M.Cancer du colon proximal et cancer du colon distal: deux cancers epidemiologiquement differents. Gastroenterol Clin Biol {dy1989}; 13: 255–259.
Beart RW, Melton LJ, Maruta M, Dockerty MB, Frydenberg HB, O'Fallon WM. Trends in right and left-sided colon cancer. Dis Colon Rectum {dy1983}; 26: 393–8.
West DW, Slattery ML, Robison LM, et al. Dietary intake and colon cancer: sex and anatomic site-specific associations. Am J Epidemiol {dy1989}; 130: 883–94.
Pocard M, Salmon RJ, Muleris M, et al. Deux colon-deux cancers? Adenocarcinomes coliques proximal ou distal: arguments en faveur d'une cancerogenese distincte. Bull Cancer {dy1995}; 82: 10–21.
SAS Institute, Inc. SAS/STAT Software: the PHREG Procedure, Version 6. Cary, NC (USA): SAS Institute, Inc., 1991; SAS Technical Report P-217.
SAS Institute, Inc. SAS/STAT User's Guide, Version 6. 4th Edition. Cary, NC (USA): SAS Institute, Inc., 1989.
Braun MM, Helzlsouer KJ, Hollis BW, Comstock GW. Colon cancer and serum vitamin D metabolite levels 10-17 years prior to diagnosis. Am J Epidemiol {dy1995}; 142: 608–11.
Cugini P, Coen G, Scavo D, et al. Circannual versus seasonal variation of longitudinally sampled 25-hydroxycholecalciferol serum levels. Biochem Med {dy1984}; 32: 22–9.
Hollis BW. Assessment of vitamin D nutritional and hormonal status: what to measure and how to do it. Calcif Tissue Int {dy1996}; 58: 4–5.
Tsang RC, Cruz M, Specker B. Vitamin D in infancy: 25-hydroxyvitamin D, an important bioactive principle in vivo in infancy? In: Norman AW, Bouillon R, Thomasset M, eds. Vitamin D, Gene Regulation, Structure-function Analysis and Clinical Application. Berlin, Germany: de Gruyter, 1991: 739–44.
Bell NH, Epstein S, Greene A, Shary J, Oexmann MJ, Shaw S. Evidence for alteration of the vitamin D-endocrine system in obese subjects. J Clin Invest {dy1985}; 76: 370–3.
Barger-Lux MJ, Heaney RP, Lanspa SJ, Healy JC, DeLuca HF. An investigation of sources of variation in calcium absorption efficiency. J Clin Endocrinol Metab {dy1995}; 80: 406–11.
Bell NH. 25-hydroxyvitamin D3 reverses alteration of the vitamin D-endocrine system in blacks. Am J Med {dy1995}; 99: 597–9.
Corder EH, Guess HA, Hulka BS, et al. Vitamin D and prostate cancer: a prediagnostic study with stored sera. Cancer Epidemiol Biomark Prev {dy1993}; 2: 467–72.
Corder EH, Friedman GD, Vogelman JH, Orentreich N. Seasonal variation in vitamin D, vitamin D-binding protein and dehydroepiandrosterone: risk of prostate cancer in black and white men. Cancer Epidemiol Biomark Prev {dy1995}; 4: 655–9.
Mawer EB, Hayes ME, Heys SE, et al. Constitutive synthesis of 1,25-dihydroxyvitamin D3 by a human small cell lung cancer cell line. J Clin Endocrinol Metab {dy1994}; 79: 554–60.
Reitsma PH, Rothberg PG, Astrin SM, et al. Regulation of c-myc gene expression in HL-60 leukemia cells by a vitamin D metabolite. Nature {dy1983}; 306: 492–6.
Matsui T, Takahashi R, Mihara K, et al. Cooperative regulation of c-myc expression in differentiation of human promyelocytic leukemia induced by recombinant gamma-interferon and 1,25-dihydroxyvitamin D3. Cancer Res {dy1985}; 45: 4366–71.
Manolagas SC, Provedini DM, Murray EJ, Murray SS, Tsonis PA, Spandidos DA. Association between the expression of the c-myc oncogene mRNA and the expression of the receptor protein for 1,25-dihydroxyvitamin D3. Proc Natl Acad Sci {dy1987}; 84: 856–60.
Brelvi ZS, Studzinski GP. Inhibition of DNA synthesis by an inducer of differentiation of leukemic cells, 1 alpha, 25-dihydroxyvitamin D3, precedes down regulation of the c-myc gene. J Cell Physiol {dy1986}; 128: 171–9.
Eisman JA, Koga M, Sutherland RL, Barkla DH, Tutton PJM. 1,25-dihydroxyvitamin D3 and the regulation of human cancer cell replication. Proc Soc Exp Biol Med {dy1989}; 191: 221–6.
Vandewalle B, Wattez N, Lefebvre J. Effects of vitamin D3 derivatives on growth, differentiation and apoptosis in tumoral colonic HT 29 cells: possible implication of intracellular calcium. Cancer Lett {dy1995}; 97: 99–106.
Lointier P, Meggouh F, Pezet D, et al. Specific receptors for 1,25-dihydroxyvitamin D3 and human colorectal carcinogenesis. Anticancer Res {dy1989}; 9: 1921–4.
Meggouh F, Lointier P, Saez S. Sex steroid and 1,25-dihydroxyvitamin D3 receptors in human colorectal adenocarcinoma and normalmucosa. Cancer Res {dy1991}; 51: 1227–33.
Lointier P, Meggouh F, Dechelotte P, et al. 1,25-dihydroxyvitamin D3 receptors and human colon adenocarcinoma. Br J Surg {dy1991}; 78: 435–9.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Tangrea, J., Helzlsouer, K., Pietinen, P. et al. Serum levels of vitamin D metabolites and the subsequent risk of colon and rectal cancer in Finnish men. Cancer Causes Control 8, 615–625 (1997). https://doi.org/10.1023/A:1018450531136
Issue Date:
DOI: https://doi.org/10.1023/A:1018450531136