Abstract
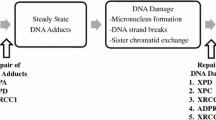
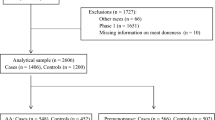
The often observed association between red meat and colorectal cancercould be due in part to mutagens, such as heterocyclic amines (HCA), that arepresent in cooked meat. HCAs are highly mutagenic and cause intestinal tumorsin animals. The hypothesis that HCAs are also carcinogenic to humans remainsto be substantiated in epidemiologic studies. We determined the associationsof meat preparation and frequency of intake (proxy variables for HCAexposure, since HCA concentration depends on the type of meat and the way itis cooked) with the prevalence of distal colorectal adenomas in asigmoidoscopy-based case-control study of 488 matched pairs of subjects fromtwo California (United States) Kaiser Permanente Medical Centers. A more thantwofold difference in adenoma prevalence between subjects at extreme ends ofestimated HCA intake was observed. For subjects who ate red meat more thanonce per week, fried it more than 10 percent of the time, and ate it with adarkly browned surface, compared with subjects who ate red meat one time orless per week, fried it 10 or less percent of the time, and ate it with alightly browned surface, the odds ratio was 2.2 (95 percent confidenceinterval = 1.1-4.3). Adenoma prevalence also increased with frequency offrying red meat (P trend = 0.004). These results are consistent with acarcinogenic effect of HCA.
Similar content being viewed by others
References
Giovannucci E, Rimm EB, Stampfer MJ, Colditz GA, Ascherio A, Willett WC. Intake of fat, meat, and fiber in relation to risk of colon cancer in men. Cancer Res 1994; 54 :2390–7.
Goldbohm RA, van den Brandt PA, van 't Veer P, et al. A prospective cohort study on the relation between meat consumption and the risk of colon cancer. Cancer Res 1994; 54 :718–23.
Steinmetz KA, Potter JD. Food-group consumption and colon cancer in the Adelaide case-control study. II. Meat, poultry, seafood, dairy foods and eggs. Int J Cancer 1993; 53 :720–7.
Bostick RM, Potter JD, Kushi LH, et al. Sugar, meat, and fat intake, and non-dietary risk factors for colon cancer incidence in Iowa women (United States). Cancer Causes Control 1994; 1: 38–52.
Kune GA, Kune S, Read A, McGowan K, Penfold C, Watson LF. Colorectal polyps, diet, alcohol, and family history of colorectal cancer: A case-control study. Nutr Cancer 1991; 16 :25–30.
Kono S, Imanishi K, Shinchi K, Yanai F. Relationship of diet to small and large adenomas of the sigmoid colon. Jpn J Cancer Res 1993; 84 :13–9.
Neugut AI, Garbowski GC, Lee WC, et al. Dietary risk factors for the incidence and recurrence of colorectal adenomatous polyps. Ann Intern Med 1993; 118 :91–5.
Giovannucci E, Stampfer MJ, Colditz G, Rimm EB, Willett WC. Relationship of diet to risk of colorectal adenoma in men. JNCI 1992; 84: 91–8.
Macquart-Moulin G, Riboli E, Cornee J, Kaaks R, Berthezene P. Colorectal polyps and diet: a case-control study in Marseille. Int J Cancer 1987; 40:179–88.
Lev R. Adenomatous Polyps of the Colon. New York, NY (USA): Springer-Verlag, 1990.
Stryker SJ, Wolff BG, Culp CE, Libbe SD, Ilstrup DM, MacCarty RL. Natural history of untreated colonic polyps. Gastroenterology 1987; 93:1009–13.
Felton JS, Knize MG. Heterocyclic-amine mutagens/ carcinogens in foods. In: Cooper CS, Grover PL, eds. Chemical Carcinogenesis and Mutagenesis I, Handbook of Experimental Pharmacology.Berlin, Germany: Springer Verlag, 1990.
Oevervik E, Gustafsson JA. Cooked-food mutagens: current knowledge of formation and biological significance. Mutagenesis 1990; 5 :437–46.
Wakabayashi K, Nagao M, Esumi H, Sugimura T. Food-derived mutagens and carcinogens. Cancer Res 1992; 52(Suppl) :2092s–98s.
Toyota M, Ushijima T, Kakiuchi H, et al. Genetic alterations in rat colon tumors induced by heterocyclic amines. Cancer 1996; 77: 1593–7.
Sugimura T. Past, present, and future of mutagens in cooked foods. Environ Health Perspectives 1986; 67 :5–10.
Layton DW, Bogen KT, Knize MG, Hatch FT, Johnson VM, Felton JS. Cancer risk of heterocyclic amines in cooked foods: an analysis and implications for research. Carcinogenesis 1995; 16 :39–52.
Sinha R, Rothman N, Brown ED, et al. High concentrations of the carcinogen 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) occur in chicken but are dependent on the cooking method. Cancer Res 1995; 55 :4516–9.
Knize MG, Shen NH, Felton JS. The production of mutagens in foods. Proc Air Pollution Control Assoc, 1988: paper 88-130.5.
Ushiyama H, Wakabayashi K, Hirose M, Ito H, Sugimura T, Nagao M. Presence of carcinogenic heterocyclic amines in urine of healthy volunteers eating normal diet, but not of inpatients receiving parental alimentation. Carcinogenesis 1991; 12 :1417–22.
Friesen MD, Kaderlik K, Lin D, et al. Analysis of DNA adducts of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine in rat and human tissues by alkaline hydrolysis and gas chromatography/electron capture mass spectrometry: validation by comparison with 32P-postlabeling. Chem Res Toxicol 1994; 7 :733–9.
Lang NP, Butler MA, Massengill J, et al. Rapid metabolic phenotypes for acetyltransferase and cytochrome p4501A2 and putative exposure to food-borne heterocyclic amines increase the risk of colorectal cancer or polyps. Cancer Epidemiol Biomark Prev 1994; 3 :675–82.
Roberts-Thomson IC, Ryan P, Khoo KK, Hart WJ, McMichael AJ, Butler RN. Diet, acetylator phenotype, and risk of colorectal neoplasia. Lancet 1996; 347: 1372–4.
Vineis P, McMichael A. Interplay between heterocyclic amines in cooked meat and metabolic phenotype in the etiology of colon cancer. Cancer Causes Control 1996; 7:479–86.
Rimm EB, Giovannucci EL, Stampfer MJ, et al. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol 1992; 135 :1114–76.
Willett WC. Nutritional Epidemiology. New York, NY (USA): Oxford University Press, 1990.
Witte JS, Longnecker MP, Bird CL, Lee ER, Frankl HD, Haile RW. Relation of vegetable, fruit, and grain consumption to colorectal adenomatous polyps. Am J Epidemiol 1996; 144: 1015–25.
Knize MG, Sinha R, Salmon CP, Mehta SS, Dewhirst KP, Felton JS. Formation of heterocyclic amine mutagens/carcinogens during cooking of muscle meats. J Muscle Foods 1996; 7: 271–9.
Greenland S. Modeling and variable selection in epidemiologic analysis. Am J Public Health 1989; 79 :340–49.
Steineck G, Gerhardsson de Verdier M, Oevervik E. The epidemiologic evidence concerning intake of mutagenic activity from the fried surface and the risk of cancer cannot justify preventive measures. Eur J Cancer Prev 1993; 2 :293–300.
Gerhardsson de Verdier M, Hagman U, Peters RK, Steineck G, Oeverik, E. Meat, cooking methods and colorectal cancer: a case-referent study in Stockholm. Int J Cancer 1991; 49 :520–5.
Schiffman MH. Fried foods and the risk of colon cancer [Letter]. Am J Epidemiol 1990; 131 :376–8.
Muscat JE, Wynder E. The consumption of well-done red meat and the risk of colorectal cancer. Am J Public Health 1994; 84:856–8.
Willett WC, Stampfer MJ, Colditz GA, Rosner BA, Speizer FE. Relation of meat, fat, and fiber intake to the risk of colon cancer in a prospective study among women. N Engl J Med 1990, 323 :1664–72.
Lyon JL, Mahoney AW. Fried foods and the risk of colon cancer. Am J Epidemiol 1988; 128 :1000–6.
Young TB, Wolf DA. Case-control study of proximal and distal colon cancer and diet in Wisconsin. Int J Cancer 1988; 42 :167–75.
Peters RK, Garabrant DH, Yu MC, Mack TM. A case-control study of occupational and dietary factors in colorectal cancer in young men by subsite. Cancer Res 1989; 49 :5449–68.
Knekt P, Steineck G, Jaervinen R, Hakulinen T, Aromaa A. Intake of fried meat and risk of cancer: a follow-up study in Finland. Int J Cancer 1994; 59 :756–60.
Gross GA, Turesky RJ, Fay LB, Stillwell WG, Skipper PL, Tannenbaum SR. Heterocyclic aromatic amine formation in grilled bacon, beef, and fish and in grill scrapings. Carcinogenesis 1993; 14 :2313–8.
Ji H, Yu MC, Stillwell WG, et al. Urinary excretion of 2-amino-3,8-dimethylimidazo-[4,5-f]quinoxaline in white, black, and Asian men in Los Angeles County. Cancer Epidemiol Biomark Prev 1994; 3 :407–11.
Knize M, Sinha R, Rothman N, et al.Heterocyclic amine content in fast-food meat products. Food Chem Toxicol 1995; 33 :545–51.
Foutch PG, Mai H, Pardy K, et al. Flexible sigmoidoscopy may be ineffective for secondary prevention of colorectal cancer in asymptomatic, average-risk men. Dig Dis Sci 1991; 36: 924–8.
Rex DK, Lehman GA, Ulbright TM, et al. Colonic neoplasia in asymptomatic persons with negative fecal occult blood tests: influence of age, gender, and family history. Am J Gastroenterol 1993; 88: 825–31.
Potter JD, Slattery ML, Bostick RM, et al. Colon cancer: a review of the epidemiology. Epidemiol Rev 1993; 499–545.
Kakiuchi H, Watanabe M, Ushuima T, et al.Specific 5'-GGGA-3'-5'-GGA-3' mutation of the Apcgene in rat colon tumors induced by 2-amino-1-methyl-6-phenylimidazo[ 4,5-b]pyridine. Proc Natl Acad Sci USA 1995; 92:910–4.
Turesky RJ, Lang NP, Butler MA, Teitel CH, Kadlubar FF. Metabolic activation of carcinogenic heterocyclic amines by human liver and colon. Carcinogenesis 1991; 12 :1839–45.
Boobis AR, Lynch AM, Murray S, et al. CYP1A2-catalyzed conversion of dietary heterocyclic amines to their proximate carcinogens is their major route of metabolism in humans. Cancer Res 1994; 54 :89–94.
Holme JA, Wallin H, Brunborg G, Soderlund EJ, Hongslo JK, Alexander J. Genotoxicity of the food mutagen 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP): formation of 2-hydroxamino-PhIP, a directly acting genotoxic metbolite. Carcinogenesis 1989; 10 :1389–96.
Yamazoe Y, Abu-Zeid M, Gong D, Staino N, Kato R. Enzymatic acetylation and sulfation of N-hydroxy heterocyclic amines in bacteria and rat livers. Carcinogenesis 1989; 10 :1675–8.
Buonarati MH, Turteltaub KW, Felton JS. Role of sulfation and acetylation in the activation of 2-hydroxyamino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) to intermediates which bind DNA. Mutat Res 1990; 245 :185–90.
Snyderwine EG, Wirth PJ, Roller PP, Adamson RH, Sato S, Thorgeirsson SS. Mutagenicity and in vitro covalent DNA binding of 2-hydroxyamino-3-methylimidazolo[ 4,5-f]quinoline. Carcinogenesis 1988; 9 :411–8.
Minchin RF, Reeves PT, Teitel CH, et al.N-and O-acetylation of aromatic and heterocyclic amine carcinogens in human monomorphic and polymorphic acetyltransferase expressed in cos-1cells. Biochem Biophys Res Commun 1992; 185 :839–44.
Chou HC, Lang NP, Kadlubar FF. Metabolic activation of N-hydroxy arylamines and N-hydroxy heterocyclic amines by human sulfotransferase(s). Cancer Res 1995; 55 :525–9.
Lin D, Meyer DJ, Ketterer B, Lang NP, Kadlubar FF. Effects of human and rat glutathione S-transferases on the covalent DNA binding of N-acetoxy derivatives of heterocyclic amine carcinogens in vitro: a possible mechanism of organ specificity in their carcinogenesis. Cancer Res 1994; 54: 4920–6.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Probst-Hensch, N.M., Sinha, R., Longnecker, M.P. et al. Meat preparation and colorectal adenomas in a large sigmoidoscopy-based case-control study in California (United States). Cancer Causes Control 8, 175–183 (1997). https://doi.org/10.1023/A:1018416128894
Issue Date:
DOI: https://doi.org/10.1023/A:1018416128894