Abstract
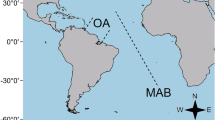
Dispersal plays an important role in the establishment and maintenance of biodiversity and, for most deep-sea benthic marine invertebrates, it occurs mainly during the larval stages. Therefore, the mode of reproduction (and thus dispersal ability) will affect greatly the biogeographic and bathymetric distributions of deep-sea organisms. We tested the hypothesis that, for bathyal and abyssal echinoderms and ascidians of the Atlantic Ocean, species with planktotrophic larval development have broader biogeographic and bathymetric ranges than species with lecithotrophic development. In comparing two groups with lecithotrophic development, we found that ascidians, which probably have a shorter larval period and therefore less dispersal potential, were present in fewer geographic regions than elasipod holothurians, which are likely to have longer larval periods. For asteroids and echinoids, both the geographic and bathymetric ranges were greater for lecithotrophic than for planktotrophic species. For these two classes, the relationships of egg diameter with geographic and bathymetric range were either linearly increasing or non-monotonic. We conclude that lecithotrophic development does not necessarily constrain dispersal in the deep sea, probably because species with planktotrophic development may be confined to regions of high detrital input from the sea surface. Our data suggest that more information is necessary on lengths of larval period for different species to accurately assess dispersal in the deep sea.
Similar content being viewed by others
References
Birkeland, C., Chia, F.-S. and Strathmann, R.R. (1971) Development, substratum selection, delay of metamorphosis and growth in the seastar Mediaster aequalis Stimpson. Biol. Bull. 141, 99–108.
Bouchet, P. and Warén, A. (1994) Ontogenetic migration and dispersal of deep-sea gastropod larvae. In Reproduction, Larval Biology and Recruitment of the Deep-Sea Benthos. (C.M. Young and K.J. Eckelbarger, eds) pp. 98–117. New York: Columbia University Press.
Chesson, P. (1985) Coexistence of competitors in spatially and temporally varying environments: a look at the combined effects of different sorts of variability. Theor. Popul. Biol. 28, 263–87.
Chevaldonné, P., Jollivet, D., Vangriesheim, A. and Desbruyères, D. (1997) Hydrothermal-vent alvinellid polychaete dispersal in the eastern Pacific. 1. Influence of vent site distribution, bottom currents, and biological patterns. Limnol. Oceanogr. 42, 67–80.
Clark, A.M. and Downey, M.E. (1992) Starfishes of the Atlantic. London: Chapman & Hall.
Clark, H.L. (1925) A Catalogue of the Recent Sea-Urchins (Echinoidea) in the Collection of the British Museum (Natural History). Oxford: Oxford University Press.
Craddock, C., Lutz, R.A. and Vrijenhoek, R.C. (1997) Patterns of dispersal and larval development of archaeogastropod limpets at hydrothermal vents in the eastern Pacific. J. Exp. Mar. Biol. Ecol. 210, 37–51.
Creasy, S., Rogers, A.D. and Tyler, P.A. (1996) A genetic comparison of two populations of the deep-sea vent shrimp Rimicaris exoculata (Decapoda: Caridea: Bresiliidae) from the Mid-Atlantic Ridge. Mar. Biol. 125, 473–83.
Desbruyères, D. and Laubier, L. (1986) Les Alvinellidae, une famille nouvelle d'annélides polychètes inféodées aux source hydrothermales sous-marines: systématique, biologie et écologie. Can. J. Zool. 64, 2227–45.
Emlet, R.B., McEdward, L.R. and Strathmann, R.R. (1987) Echinoderm larval ecology viewed from the egg. In Echinoderm Studies, Vol. 2. (M. Jangoux and J.M. Lawrence, eds) pp. 55–136. Rotterdam: A.A. Balkema.
Etter, R.J. and Rex, M.A. (1990) Population differentiation decreases with depth in deep-sea gastropods. Deep-Sea Res. 37, 1251–61.
Etter, R.J. and Caswell, H. (1994) The advantages of dispersal in a patchy environment: effects of disturbance in a cellular automaton model. In Reproduction, Larval Biology and Recruitment of the Deep-Sea Benthos (C.M. Young and K.J. Eckelbarger, eds) pp. 284–305. New York: Columbia University Press.
Gage, J.D., Billet, D.S.M., Jensen, M. and Tyler, P.A. (1985) Echinoderms of the Rockall Trough and adjacent areas 2. Echinoidea and Holothurioidea. Bull. Br. Mus. Nat. Hist. (Zool.) 48, 173–213.
Gebruk, A., Tyler, P.A. and Billett, D.S.M. (in press) Pelagic juveniles of the deep-sea elasipodid holothurians: new records and review. Ophelia.
Hansen, B. (1975) Systematics and biology of the deep-sea holothurians Part 1. Elasipoda. Galathea Rep. 13, 5–262.
Hansen, T.A. (1978) Larval dispersal and species longevity in Lower Tertiary gastropods. Science 199, 885–7.
Herdman, W.A. (1882) Tunicata Part I. In Report on the Scientific Results of the Challenger Expedition during the years 1873–1876 vol. 6, pt 17 (C.W. Thomson and J. Murray, eds) pp. 1–296.
Hessler, R.R. and Thistle, D. (1975) On the place of origin of deep-sea isopods. Mar. Biol. 32, 155–65.
Hessler, R.R., Wilson, G.D. and Thistle D. (1979) The deep-sea isopods: a biogeographic and pylogenetic review. Sarsia 64, 67–75.
Jablonski, D. (1982) Evolutionary rates and modes in Late Cretaceous gastropods: role of larval ecology. Third North American Paleontol. Conv. Proceed. 1, 257–62.
Jollivet, D., Desbruyères, D., Bonhomme, F. and Moraga, D. (1995) Genetic differentiation of deep-sea hydrothermal vent alvinellid populations (Annelida: Polychaeta) along the East Pacific Rise. Heredity (Lond.) 74, 376–91.
Josefson, A. (1985) Distribution of diversity and functional groups of marine benthic infauna in the Skagerrak (eastern North Sea). Can larval availability affect diversity? Sarsia 70, 229–49.
Levin, S.A. (1974) Dispersion and population interactions. Am. Nat. 108, 207–28.
Madsen, F.J. (1961) On the zoogeography and origin of the abyssal fauna in view of the knowledge of the Porcellanasteridae. Galathea Rep. 4, 177–218.
Menzies, R.J., George, R.Y. and Rowe, G.T. (1973) Abyssal Environment and Ecology of the World Oceans. New York: John Wiley & Sons.
Monniot, C. and Monniot, F. (1973) Ascidies abyssales récoltées au cours de la campagne océanographique Biaçores par le “Jean-charcot”. Bull. Mus. Nat. Hist. Natur. 121, 389–475.
Mortensen, T. (1927) Handbook of the Echinoderms of the British Isles. London: Oxford University Press.
Mortensen, T. (1928) A Monograph of the Echinoidea, Vol. I, Cidaroidea. Copenhagen: C.A. Reitzel.
Mortensen, T. (1935) A Monograph of the Echinoidea, Vol. II, Bothriocidaroidea, Melonechinoida, Lepidocentroida & Stirodonta. Copenhagen: C.A. Reitzel.
Mortensen, T. (1940) A Monograph of the Echinoidea, Vol. III (1) Aulondonta. Copenhagen: C.A. Reitzel.
Mortensen, T. (1943a) A Monograph of the Echinoidea, Vol. III (2) Camarodonta (1). Copenhagen: C.A. Reitzel.
Mortensen, T. (1943b) A Monograph of the Echinoidea, Vol. III (3) Camarodonta (2). Copenhagen: C.A. Reitzel.
Mortensen, T. (1948a) A Monograph of the Echinoidea, Vol. IV (1) Holectypoida and Cassiduloida. Copenhagen: C.A. Reitzel.
Mortensen, T. 1948b) A Monograph of the Echinoidea, Vol. IV (2) Clypeastroida. Copenhagen: C.A. Reitzel.
Mortensen, T. (1950) A Monograph of the Echinoidea, Vol. V (1) Spatangoida 1. Copenhagen: C.A. Reitzel.
Mortensen, T. (1951) A Monograph of the Echinoidea, Vol. V (2) Spatangoida 2. Copenhagen: C.A. Reitzel.
Moseley, H.N. (1880) Deep-sea dredgings and life in the deep sea. Nature 21, 543–7.
Olson, R.R. and Olson, M.H. (1989) Food limitation of planktotrophic marine invertebrate larvae: does it control recruitment success? Ann. Rev. Ecol. Syst. 20, 225–47.
Palmer, A.R. and Strathmann, R.R. (1981) Scale of dispersal in varying environments and its implications for life-histories of marine invertebrates. Oecologia 48, 308–18.
Pearse, J.S. (1994) Cold-water echinoderms break “Thorson's rule”. In Reproduction, Larval Biology and Recruitment of the Deep-Sea Benthos (C.M. Young and K.J. Eckelbarger, eds) pp. 26–43. New York: Columbia University Press.
Pineda, J. (1993) Boundary effects on the vertical ranges of deep-sea benthic species. Deep-Sea Res. 40, 2179–92.
Rex, M.A. (1981) Community structure in the deep-sea benthos. Ann. Rev. Ecol. Syst. 12, 331–53.
Rex, M.A. (1983) Geographic patterns of species diversity in the deep-sea benthos. In Deep Sea Biology (G.T. Rowe, ed.) pp. 453–72. New York: John Wiley & Sons.
Rex, M.A. and Warén, A. (1982) Planktotrophic development in deep-sea prosobranch snails from the western North Atlantic. Deep-Sea Res. 29, 171–84.
Rex, M.A., Stuart, C.T., Hessler, R.R., Allen, J.A., Sanders, H.L. and Wilson, G.D.F. (1993) Global-scale latitudinal patterns of species diversity in the deep-sea benthos. Nature 365, 636–9.
Sanders, H.L. (1979) Evolutionary ecology and life-history patterns in the deep sea. Sarsia 64, 1–7.
Sanders, H.L. and Grassle, J.F. (1971) The interactions of diversity, distribution and mode of reproduction among major grouping of deep-sea benthos. Proc. Joint Oceanogr. Assembl. (Tokyo, 1970) 260–2.
Scheltema, R.S. (1986) On dispersal and planktonic larvae of benthic invertebrates; an eclectic overview and summary of problems. Bull. Mar. Sci. 39, 290–322.
Serafy, D.K. (1979) Echinoids (Echinodermata: Echinoidea). Memoirs of the Hourglass Cruises 5, 1–120.
Shilling, F.M. and Manahan, D.T. (1994) Energy metabolism and amino acid transport during early development of Antarctic and temperate echinoderms. Biol. Bull. 187, 398–407.
Smith, C.R. and Hessler, R.R. (1987) Colonization and succession in deep-sea ecosystems. Trends Ecol. Evol. 2, 359–63.
Strathmann, R.R. (1974) The spread of sibling larvae of sedentary marine invertebrates. Am. Nat. 108, 29–44.
Stuart, C.T. and Rex, M.A. (1994) The relationship between developmental pattern and species diversity in deep-sea prosobranch snails. In Reproduction, Larval Biology and Recruitment of the Deep-Sea Benthos (C.M. Young and K.J. Eckelbarger, eds) pp. 118–36. New York: Columbia University Press.
Svane, I. and Young, C.M. (1989) The ecology and behaviour of ascidian larvae. Oceanogr. Mar. Biol. Annu. Rev. 27, 45–90.
Thorson, G. (1950) Reproduction and larval ecology of marine bottom invertebrates. Biol. Rev. 25, 1–45.
Tyler, P.A. (1995) Conditions for the existence of life at the deep-sea floor: an update. Oceanogr. Mar. Biol. Ann. Rev. 33, 221–44.
Tyler, P.A. and Young, C.M. (1992) Reproduction in marine invertebrates in “stable” environments: the deep sea model. Invert. Reprod. Develop. 22, 185–92.
Tyler, P.A., Young, C.M. and Serafy, K. (1995) Distribution, diet and reproduction in the genus Echinus: Evidence for recent diversification? In Echinoderm Research 1995 (R.H. Emson, A.B. Smith and A.C. Campbell, eds) pp. 29–35. Rotterdam: A.A. Balkema.
Vinogradova, N.G. (1959) The zoogeographical distribution of the deep-water bottom fauna in the abyssal zone of the ocean. Deep-Sea Res. 5, 205–8.
Wilson, G.D.F. and Hessler, R.R. (1987) Speciation in the deep sea. Ann. Rev. Ecol. Syst. 18, 185–207.
Young, C.M. (1992) Episodic recruitment and cohort dominance in echinoid populations at bathyal depths. In Marine Eutrophication and Population Dynamics, Proceedings of the 25th EMBS (G. Colombo et al., eds) pp. 239–46. Fredensborg: Olsen & Olsen.
Young, C.M. (1994) A tale of two dogmas: the early history of deep-sea reproductive biology. In Reproduction, Larval Biology and Recruitment of the Deep-Sea Benthos (C.M. Young and K.J. Eckelbarger, eds) pp. 1–25. New York: Columbia University Press.
Zal, F., Jollivet, D., Chevaldonné and Desbruyères, D. (1995) Reproductive biology and population structure of the deep-sea hydrothermal vent worm Paralvinella grasslei (Polychaeta: Alvinellidae) at 13°N on the East Pacific Rise. Mar. Biol. 122, 637–48.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Young, C.M., Sewell, M.A., Tyler, P.A. et al. Biogeographic and bathymetric ranges of Atlantic deep-sea echinoderms and ascidians: the role of larval dispersal. Biodiversity and Conservation 6, 1507–1522 (1997). https://doi.org/10.1023/A:1018314403123
Issue Date:
DOI: https://doi.org/10.1023/A:1018314403123