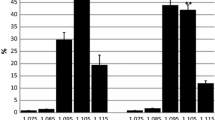
Abstract
The activities of the enzymes related to the malate–aspartate shuttle, which convert cytosolic NADH into mitochondrial NADH, were measured in red and white blood cells from thoroughbred horses undergoing continuous training (race horses) and compared with those in blood cells from riding horses. The activities of malate dehydrogenase (MDH), a rate-limiting enzyme for the malate-aspartate shuttle, were significantly elevated in the white blood cells (WBC) from race horses compared with those from riding horses. There were no significant differences in the activities of the enzymes in the red blood cells between race horses and riding horses. This increase in the MDH activity in their WBC is considered to reflect the increased metabolic activity in the race horses resulting from the training.
Similar content being viewed by others
REFERENCES
Arai, T., Machida, Y., Sasaki, M. and Oki, Y., 1989. Hepatic enzyme activities and plasma insulin concentrations in diabetic herbivorous voles. Veterinary Research Communications, 13, 421-426
Arai, T., Washizu, T., Hamada, S., Sako, T., Takagi, K., Yashiki, K. and Motoyoshi, S., 1994. Glucose transport and glycolytic enzyme activities in erythrocytes of two-year-old thoroughbreds undergoing training exercise. Veterinary Research Communications, 18, 417-422
Arai, T., Sugawara, M., Sako, T. and Motoyoshi, S., 1996. Within-day changes in blood glucose and plasma insulin concentrations and erythrocytes enzyme activities in race horses at rest. Pferdeheilkunde, 12, 485-487
Arai, T., Kawaue, T., Abe, M., Kuramoto, E., Nuruki, R. and Sako, T., 1997. Glycolytic enzyme activities in leukocytes of Thoroughbreds undergoing training exercise. Journal of Equine Science, 8, 113-116
Arai, T., Kawaue, T., Abe, M., Kuramoto, E., Kawakami, E., Sako, T. and Washizu, T., 1998. Comparison of glucokinase activities in the peripheral leukocytes between dogs and cats. Comparative Biochemistry and Physiology, 120C, 53-56
Bradford, M.M., 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72, 248-254
Eto, K., Tsubamoto, Y., Terauchi, Y., Sugiyama, T., Kishimoto, T., Takahashi, N., Yamauchi, N., Kubota, N., Maruyama, S., Aizawa, T., Akanuma, Y., Aizawa, S., Kasai, H., Yazaki, Y. and Kadowaki, T., 1999. Role of NADH shuttle system in glucose-induced activation of mitochondrial metabolism and insulin secretion. Science, 283, 981-985
Hedeskov, C.J., Capito, K. and Thams, P., 1987. Cytosolic ratios of free [NADPH]/[NADP+] and [NADH]/[NAD+] in mouse pancreatic islets, and nutrient-induced insulin secretion. Biochemical Journal, 241, 161-167
Huggett, A.G. and Nixon, D.A., 1957. Use of glucose oxidase, peroxidase and o-dianisidine in determination of blood and urinary glucose. Lancet, ii, 368-370
Kaloustian, H.D., Stolzenbach, F.E., Everse, J. and Kaplan, N.O., 1969. Lactate dehydrogenase of lobster (Hornarus americanus) tail muscle I. Physical and chemical properties. Journal of Biological Chemistry, 244, 2891-2901
MacDonald, M.J., 1981. High content of mitochondrial glycerol-3-phosphate dehydrogenase in pancreatic islet and its inhibition by diazoxide. Journal of Biological Chemistry, 256, 8287-8290
MacDonald, M.J., 1982. Evidence of the malate aspartate shuttle in pancreatic islet. Archives Biochemistry and Biophysics, 213, 643-649
Matschinsky, F.M., 1996. A lesson in metabolic regulation inspired by the glucokinase glucose sensor paradigm. Diabetes, 45, 223-241
Rej, R. and Horder, M., 1983. Aspartate aminotransferases (glutamate oxaloacetate transaminase). In: H.U. Bergmeyer (ed.), Methods of Enzymatic Analysis, 3rd edn, vol. 3 (VCH, New York), 416-433
Schmidt, E., 1974. Glutamate dehydrogenase UV-assay. In: H.U. Bergmeyer (ed.), Methods of Enzymatic Analysis, vol. 2, (Academic Press, New York), 650-656
Siegel, E. and Bing, R.J., 1956. Plasma enzyme activity in myocardial infarction in dogs and man. Proceedings of the Society for Experimental Biology and Medicine, 91, 604-607
Washizu, T., Kuramoto, E., Abe, M., Sako, T. and Arai, T., 1998. A comparison of the activities of certain enzymes related to energy metabolism in leukocytes in dogs and cats. Veterinary Research Communications, 22, 187-192
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Arai, T., Takahashi, M., Araki, K. et al. Activities of Enzymes Related to the Malate–Aspartate Shuttle in the Blood Cells of Thoroughbred Horses Undergoing Training Exercise. Vet Res Commun 25, 577–583 (2001). https://doi.org/10.1023/A:1017977200420
Issue Date:
DOI: https://doi.org/10.1023/A:1017977200420