Abstract
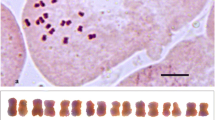
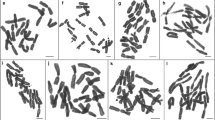
The diploid chromosome number of the cotton boll weevil, Anthonomus grandis Boheman, is 44. Both C‐ and N‐banding techniques of mitotic cells demonstrated constitutive heterochromatin in the p arm of the eight largest chromosomes, the p arm of the X chromosome, and the centromeric region of autosomal groups A–D. Neither the y nor the group E autosomes appeared to contain constitutive heterochromatin. Supernumerary chromosomes were not found in the boll weevil. Restriction endonuclease banding of primary spermatocytes revealed a rod‐shaped Xy tetrad in which the X and y were terminally associated. The p arm of the large, submetacentric X was C‐band positive. While two of the autosomal tetrads were typically ring‐shaped in primary spermatocytes, the remaining 19 autosomal tetrads were rod‐shaped.
Similar content being viewed by others
References
Asana, J. J., S. Makino & H. Niyama, 1942. A chromosomal survey of some Indian insects IV. On the sex chromosomes of some species of beetles (Coleoptera). Cytologia 12:187–205.
Camacho, J.P.M., J. Cabrero, E. Viseras, M.D. Lopez-Leon, J. Navas-Castillo & J.D. Alche, 1991. G banding in two species of grasshopper and its relationship to C, N, and fluorescence banding techniques. Genome 34:638–643.
Colomba, M.S., R. Vitturi & M. Zunino, 2000. Karyotype analysis, banding, and fluorescent in situ hybridization in the scarab beetle Gymnopleurus sturmi McLeay (Coleoptera Scarabaeoidea: Scarabaeidae). J. Hered. 91: 260–264.
Dolfini, S.F., 1990. Heterochromatin heterogeneity in Drosophila chromosomes detected by Alu I-banding. Hereditas 112: 141–149.
Ennis, T.J., 1974. Chromosome structure in Chilocorus (Coleoptera: Coccinellidae). I. Fluorescent and giemsa banding patterns. Can. J. Genet. Cytol. 16: 651–661.
Ennis, T.J., 1975. Feulgen hydrolysis and chromosome banding in Chilocorus (Coleoptera: Coccinellidae). Can. J. Genet. Cytol. 17: 75–80.
Fenocchio, A.S. & L.A.C. Bertollo, 1990. Supernumerary chromosomes in a Rhamdia hilarii population (Pisces, Pimelodidae). Genetica 81: 193–198.
Foresti, F., L.F. Almeida-Toledo & S.A. Toledo, 1989. Supernumerary chromosome system, C-banding pattern characterization, and multiple nucleolus organizer regions in Moenkhausia sanctaefilomenae (Pisces, Characidae). Genetica 79: 107–114.
Fox, D.P. & J.L. Santos, 1985. N-bands and nucleolus expression in Schistocerca gregaria and Locusta migratoria. Heredity 54: 333–341.
Funaki, K., S. Matsui & M. Sasaki, 1975. Location of nucleolar organizers in animal and plant chromosomes by means of an improved N-banding technique. Chromosoma 49: 357–370.
Garagna, S., A. Perez-Zapata, M. Zuccotti, S. Mascheretti, N. Marziliano, C.A. Redi, M. Aguilera, & E. Capanna, 1997. Genome composition in Venezuelan spiny-rats of the genus Proechimys (Rodentia, Echimyidae). I. Genome size, C-heterochromatin and repetitive DNAs in situ hybridization patterns. Cytogenet. Cell Genet. 78: 36–43.
Gosalvez, J., J.L. Bella, C. Lopez-Fernandez & R. Mezzanotte, 1987. Correlation between constitutive heterochromatin and restriction enzyme resistant chromatin in Arcyptera tornosi (Orthoptera). Heredity 59: 173–180.
Green, D.M., 1990. Muller's ratchet and the evolution of supernumerary chromosomes. Genome 33: 818–824.
Guest, W.C. & T.C. Hsu, 1973. A new technique for preparing Drosophila neuroblast chromosomes. Drosophila Inf. Serv. 50: 193.
Kaelbling, M., D.A. Miller & O.J. Miller, 1984. Restriction enzyme banding of mouse metaphase chromosomes. Chromosoma 90: 128–132.
Lloyd, M.A. & G.H. Thorgaard, 1988. Restriction endonuclease banding of rainbow trout chromosomes. Chromosoma 96: 171–177.
Lorite, P., A.E. Arangea, F. Luque & T. Palomeque, 1997. Analysis of the nucleolar organizing regions in the ant Tapinoma nigerrimum. Heredity 78: 578–582.
Lue, P.S., J.E. Watson & F.R. Gilliland, Jr., 1973. Karyology of the boll weevil. Ann. Entomol. Soc. Am. 66: 801–802.
Lue, P.S., J.E. Watson & F.R. Gilliland Jr., 1976. Karyotypic determination in the boll weevil. J. Hered. 67: 308–312.
Mahan, J.T. & M.L. Beck, 1986. Heterochromatin in mitotic chromosomes of the Virilis species group of Drosophila. Genetica 68: 113–118.
Marchi, A. & R. Mezzanotte, 1988. Restriction endonuclease digestion and chromosome banding in the mosquito, Culiseta longiareolata (Diptera: Culicidae). Heredity 60: 21–26.
Manicardi, G.C., D. Bizzaro, M. Mandrioli & U. Bianchi, 1998. Silver staining as a new banding technique to aphid chromosomes. Chromosome Res. 6: 55–57.
Mezzanotte, R., P.E. Manconi & L. Ferrucci, 1986. On the possibility of localizing in situ Mus musculus and Drosophila virilis satellite DNAs by Alu I and EcoRI restriction endonucleases. Genetica 70: 107–111.
National Cotton Council Bulletin, 1994. Boll Weevil Eradication: A National Strategy for Success. National Cotton Council, Memphis, TN, pp. 1–13.
North, D.T., R.A. Leopold & D. Childress, 1981. Meiotic and mitotic chromosomes of the cotton boll weevil (Coleoptera: Curculionidae). Can. J. Genet. Cytol. 23: 443–447.
Padilla, J.A., J.L. Fernandez-Garcia, A. Rabasco, M. Martinez-Trancon, I. Rodriguez de Ledsma & J.J. Perez-Regadera, 1993. Characterization of the karyotype of the tench (Tinca tinca L.) and analyzation of its chromosomal heterochromatic regions by C-banding, Ag-staining, and restriction endonuclease banding. Cytogenet. Cell Genet. 62: 220–223.
Palomeque, T., E. Chica, M.A. Cano & R. Diaz de la Guadria, 1988. Karyotypes, C-banding, and chromosomal location of active nucleolar organizing regions in the Tapinoma (Hymenoptera, Formicidae). Genome 30: 277–280.
Palomeque, T., E. Chica & R. Diaz de la Guadria, 1990. Karyotype, C-banding, chromosomal location of active nucleolar organizing regions, and B-chromosomes in Lasius niger (Hymenoptera, Formicidae). Genome 33: 267–272.
Palomeque, T., E. Chica & R. Diaz de la Guardia, 1993. Supernumerary chromosome segments in different genera of Formicidae. Genetica 90: 17–29.
Pieczarka, J.C., C.Y. Nagamachi, R.M.S. Barros & M.S. Mattevi, 1996. Analysis of constitutive heterochromatin by fluorochromes and in situ digestion with restriction enzymes in species of the group Callithrix argentata (Callitrichidae, Primates). Cytogenet. Cell Genet. 72: 325–330.
Pimpinelli, S., G. Santini & M. Gatti, 1976. Characterization of Drosophila heterochromatin. Chromosoma 57: 377–386.
Rufas, J.S., J. Gosalvez, C. Lopez-Fernandez & H. Cardoso, 1983. Complete dependence between Ag, NORs and C-positive heterochromatin revealed by simultaneous Ag-NOR C-banding method. Cell Biol. Int. Rep. 7: 275–281.
Smith, S.G., 1949. Evolutionary changes in the sex chromosomes of Coleoptera. I. Wood borers of the genus Agrilus. Evolution 3: 344–357.
Smith, S.G., 1952a. The cytology of Sitpophilus (Calandra) oryzae (L.), S. granarius (L.), and some other Rhynchophora (Coleoptera). Cytologia 17: 50–70.
Smith, S.G., 1952b. The evolution of the heterochromatin in the genus Tribolium (Tenebriobnidae: Coleoptera). Chromosoma 4: 585–610.
Smith, S.G., 1953. Chromosome numbers of Coleoptera. Heredity 7: 31–48.
Smith, S.G., 1960. Chromosome numbers of Coleoptera. II. Can. J. Genet. Cytol. 2: 66–88.
Smith, S.G., 1966. Natural hybridization in the Coccinellid genus Chilocorus. Chromosoma 18: 380–406.
Sitngo, V., L. Rocco, G. Odierna & M. Bellitti, 1995. NOR and heterochromatin analysis in two cartilaginous fishes by C-, Ag-and RE (restriction endonuclease)-banding. Cytogenet. Cell Genet. 71: 228–234.
Stock, A.D., D.B. Burnham & T.C. Hsu, 1972. Giemsa banding of meiotic chromosomes with description of a procedure for cytological preparation from solid tissue. Cytogenetics 11: 534–539.
Sumner, A.R., H.J. Evans & R.A. Buckland, 1971. A new technique for distinguishing between human chromosomes. Nature 232: 31–32.
Takenouchi, Y., 1958. Further survey of the chromosomes in Curculionid weevils (Coleoptera). Jap. J. Genet. 33: 163–175.
Takenouchi, Y., 1974. A study of the chromosomes of thirtyfour species of Japanese weevils (Coleoptera: Curculionidae). Genetica 45: 91–110.
USDA., 1999. West Delta Cooperative Boll Weevil Eradication Program. Environmental Assessment. pp. 1–20.
Vahidy, A.A., Q. Jahan & B. Jahan, 1993. Giemsa N-banding polymorphism in six botanical varieties and six cultivars of barley, Hordeum vulgare L. Cytologia 58: 273–279.
Van Den Bussche, R.A., R.L. Honeycutt & R.J. Baker, 1992. Restriction endonuclease digestion patterns of harvest mice (Reithrodontomys) chromosomes: a comparison to G-bands, C-bands, and in situ hybridization. Genetica 87: 141–149.
Vinas, A., C. Gomez, P. Martinez & L. Sanchez, 1994. Induction of G-bands on Anguilla anguilla chromosomes by the restriction endonucleases HaeIII, HinfI, and MseI. Cytogenet. Cell Genet. 65: 79–81.
Wise, D., J.E. Wright & J.R. McCoy, 1982. Meiotic chromosomes of the boll weevil. J. Hered. 73: 234–236.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
McNally, L., Beck, M. & Biggers, C. Karyotypic analysis of the cotton boll weevil, Anthonomus grandis Boheman. Genetica 109, 219–225 (2000). https://doi.org/10.1023/A:1017573403380
Issue Date:
DOI: https://doi.org/10.1023/A:1017573403380